Reports
Reports
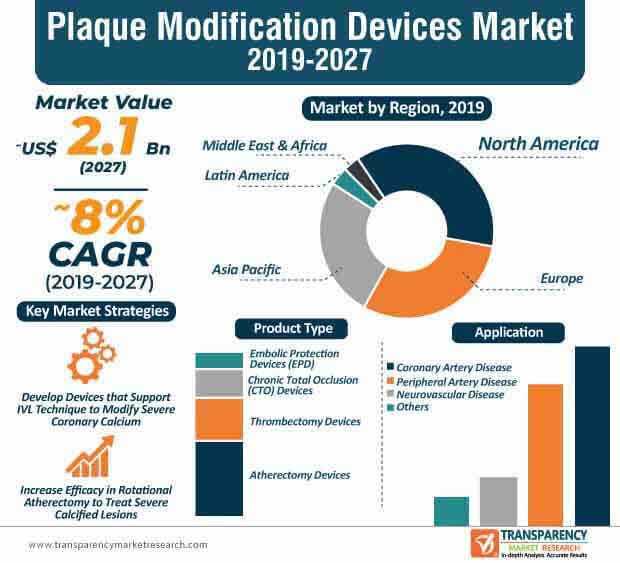
The high prevalence of severe coronary calcium among individuals has triggered the demand for plaque modification devices. As such, non-balloon and balloon-based technologies are gaining importance in the management of severe calcified lesions. Moreover, the availability of new devices are boosting growth of the plaque modification devices market, which is anticipated to reach a revenue of ~US$ 2.1 Bn by the end of 2027. Hence, healthcare companies are increasing their R&D in lithoplasty balloon that involves the use of high-energy mechanical pulses to crack coronary calcium.
Coronary lithoplasty is acquiring popularity as an easy and effective technique to alleviate deep calcium in the plaque modification devices market. Thus, advantages of coronary lithoplasty are anticipated to revolutionize the management of severe calcified coronary lesions. In order to support this trend, healthcare providers are growing increasingly aware about intravascular imaging that helps clinicians to opt for the most appropriate plaque modification devices. Novel imaging techniques help clinicians to analyze the optimal stent result.
Innovations in atherectomy devices are expected to bring about a change in the plaque modification devices market. For instance, Avinger Inc.— a commercial-stage medical device company, announced the FDA clearance for its next-gen Pantheris Lumivascular atherectomy system. This explains why atherectomy devices product type segment is estimated to lead the plaque modification devices market.
Companies in the plaque modification devices market are increasing research to develop innovative image-guided atherectomy devices for the treatment of peripheral artery diseases. Likewise, the lumivascular technology is being highly publicized for real-time intravascular imaging and is being combined with highly effective catheters for management of peripheral artery diseases. As such, companies in the market for plaque modification devices are tapping opportunities in North America due to the high prevalence of peripheral artery diseases in the U.S. They are increasing efficacy to make design improvements in atherectomy devices with the help of simplified single balloon system.
Optimal coronary stent implantation is found to be among the few successful strategies that prevent stent failures. However, there is a growing need for increased understanding of the intravascular milieu for optimization of percutaneous coronary intervention (PCI). Hence, companies are taking efforts to gain an understanding about intravascular ultrasound and optical coherence tomography (OCT) that offers insights on calcium thickness.
The plaque modification devices market is expected to expand at a striking CAGR of ~8% during the forecast period. As such, the success of plaque modification devices is highly dependent on OCT-rendered evaluation of cross-sectional calcium distribution. Likewise, preintervention OCT imaging provides accurate measurements to healthcare providers about the minimal lumen area and lesion length. Thus, innovative imaging techniques are bolstering the growth of the plaque modification devices market. Preintervention OCT imaging is generating incremental opportunities for companies in the market for plaque modification devices, since the technique is used for procedural planning and stent sizing.
Companies in the plaque modification devices market are developing devices that treat complex lesions. For instance, Boston Scientific— a U.S.-based manufacturer of medical devices for interventional medical specialties, has a broad product portfolio in plaque modification devices such as the Flextome™ Cutting Balloon™ Dilatation Device. Manufacturers are developing novel devices that help clinicians in treating resistant lesions. They are making product improvements with nylon balloon materials designed to offer flexibility and enhanced puncture resistance.
Precision dilation and superior compliance are key focus points for companies in the plaque modification devices market. On the other hand, manufacturers are innovating in rotational atherectomy devices. Manufacturers are increasing their production capacities to develop rotational atherectomy devices that are gaining global recognition. Rotational atherectomy devices help to successfully ablate calcium in coronary arteries. Likewise, these novel devices are gaining popularity in conducting safe ablation. Manufacturers are increasing research to offer precise control in order to selectively ablate calcium, whilst preserving the elastic tissue of the vessel wall.
Analysts’ Viewpoint
The expansion of product portfolio in atherectomy devices creates revenue streams for companies in the plaque modification devices market. As such, companies are aiming at FDA clearance of devices to bolster their credibility in the global landscape. Stable rotation and size flexibility are increasingly becoming the key focus points in the development of rotational atherectomy devices.
Coronary lithoplasty is gaining importance as an effective technique for the treatment of severe coronary calcium. However, difficult device delivery and suboptimal stent expansion are some of the restraints that are likely to slow down market growth. Hence, companies should increase awareness about preintervention OCT imaging that helps physicians to select optimum plaque modification strategy prior to stent implantation.
Plaque modification devices market to reach a revenue of ~US$ 2.1 Bn by 2027
Plaque modification devices market is driven by rise in prevalence of atherosclerosis, coronary & peripheral artery diseases, and hypertension
North America accounted for major share of the global plaque modification devices market owing to the increase in prevalence of atherosclerosis
The coronary artery diseases segment accounted for significant share of the plaque modification devices market
Key players in the global plaque modification devices market include Boston Scientific Corporation, B. Braun Melsungen, Becton, Dickinson and Company, Cardinal Health, Medtronic
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Plaque Modification Devices Market
4. Market Overview
4.1. Introduction
4.1.1. Market Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Plaque Modification Devices Market Analysis and Forecast, 2017–2027
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Technological Advancements
5.2. Disease Prevalence & Incidence Rate globally with Key countries
5.3. Reimbursement Scenario, by Region/globally
5.4. Growing Minimal Invasive Surgeries
6. Global Plaque Modification Devices Market Analysis and Forecast, by Product Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Plaque Modification Devices Market Value Forecast, by Product Type , 2017–2027
6.3.1. Atherectomy Devices
6.3.2. Thrombectomy Devices
6.3.3. Chronic Total Occlusion (CTO) Devices
6.3.4. Embolic Protection Devices (EPD)
6.4. Market Attractiveness Analysis, by Product Type
7. Global Plaque Modification Devices Market Analysis and Forecast, by Application
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Plaque Modification Devices Market Value Forecast, by Application, 2017–2027
7.3.1. Coronary Artery Disease
7.3.2. Peripheral Artery Disease
7.3.3. Neurovascular Disease
7.3.4. Others
7.4. Market Attractiveness Analysis, by Application
8. Global Plaque Modification Devices Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Plaque Modification Devices Market Value Forecast, by End-user, 2017–2027
8.3.1. Hospitals
8.3.2. Specialty Clinics
8.3.3. Ambulatory Surgical Centers
8.4. Market Attractiveness Analysis, by End-user
9. Global Plaque Modification Devices Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Plaque Modification Devices Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Plaque Modification Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. North America Plaque Modification Devices Market Value Forecast, by Product Type , 2017–2027
10.2.1. Atherectomy Devices
10.2.2. Thrombectomy Devices
10.2.3. Chronic Total Occlusion (CTO) Devices
10.2.4. Embolic Protection Devices (EPD)
10.3. North America Plaque Modification Devices Market Value Forecast, by Application , 2017–2027
10.3.1. Coronary Artery Disease
10.3.2. Peripheral Artery Disease
10.3.3. Neurovascular Disease
10.3.4. Others
10.4. North America Plaque Modification Devices Market Value Forecast, by End-user, 2017–2027
10.4.1. Hospitals
10.4.2. Specialty Clinics
10.4.3. Ambulatory Surgical Centers
10.5. North America Plaque Modification Devices Market Value Forecast, by Country/Sub-region, 2017–2027
10.5.1. U.S.
10.5.2. Canada
10.6. North America Plaque Modification Devices Market Attractiveness Analysis
10.6.1. By Product Type
10.6.2. By Application
10.6.3. By End-user
10.6.4. By Country
11. Europe Plaque Modification Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Europe Plaque Modification Devices Market Value Forecast, by Product Type , 2017–2027
11.2.1. Atherectomy Devices
11.2.2. Thrombectomy Devices
11.2.3. Chronic Total Occlusion (CTO) Devices
11.2.4. Embolic Protection Devices (EPD)
11.3. Europe Plaque Modification Devices Market Value Forecast, by Application, 2017–2027
11.3.1. Coronary Artery Disease
11.3.2. Peripheral Artery Disease
11.3.3. Neurovascular Disease
11.3.4. Others
11.4. Europe Plaque Modification Devices Market Value Forecast, by End-user, 2017–2027
11.4.1. Hospitals
11.4.2. Specialty Clinics
11.4.3. Ambulatory Surgical Centers
11.5. Europe Plaque Modification Devices Market Value Forecast, by Country/Sub-region, 2017–2027
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6. Europe Plaque Modification Devices Market Attractiveness Analysis
11.6.1. By Product Type
11.6.2. By Application
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Plaque Modification Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Asia Pacific Plaque Modification Devices Market Value Forecast, by Product Type , 2017–2027
12.2.1. Atherectomy Devices
12.2.2. Thrombectomy Devices
12.2.3. Chronic Total Occlusion (CTO) Devices
12.2.4. Embolic Protection Devices (EPD)
12.3. Asia Pacific Plaque Modification Devices Market Value Forecast, by Application, 2017–2027
12.3.1. Coronary Artery Disease
12.3.2. Peripheral Artery Disease
12.3.3. Neurovascular Disease
12.3.4. Others
12.4. Asia Pacific Plaque Modification Devices Market Value Forecast, by End-user, 2017–2027
12.4.1. Hospitals
12.4.2. Specialty Clinics
12.4.3. Ambulatory Surgical Centers
12.5. Asia Pacific Plaque Modification Devices Market Value Forecast, by Country/Sub-region, 2017–2027
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Asia Pacific Plaque Modification Devices Market Attractiveness Analysis
12.6.1. By Product Type
12.6.2. By Application
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America Plaque Modification Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Latin America Plaque Modification Devices Market Value Forecast, by Product Type , 2017–2027
13.2.1. Atherectomy Devices
13.2.2. Thrombectomy Devices
13.2.3. Chronic Total Occlusion (CTO) Devices
13.2.4. Embolic Protection Devices (EPD)
13.3. Latin America Plaque Modification Devices Market Value Forecast, by Application, 2017–2027
13.3.1. Coronary Artery Disease
13.3.2. Peripheral Artery Disease
13.3.3. Neurovascular Disease
13.3.4. Others
13.4. Latin America Plaque Modification Devices Market Value Forecast, by End-user, 2017–2027
13.4.1. Hospitals
13.4.2. Specialty Clinics
13.4.3. Ambulatory Surgical Centers
13.5. Latin America Plaque Modification Devices Market Value Forecast, by Country/Sub-region, 2017–2027
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Latin America Plaque Modification Devices Market Attractiveness Analysis
13.6.1. By Product Type
13.6.2. By Application
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Plaque Modification Devices Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Middle East & Africa Plaque Modification Devices Market Value Forecast, by Product Type , 2017–2027
14.2.1. Atherectomy Devices
14.2.2. Thrombectomy Devices
14.2.3. Chronic Total Occlusion (CTO) Devices
14.2.4. Embolic Protection Devices (EPD)
14.3. Middle East & Africa Plaque Modification Devices Market Value Forecast, by Application, 2017–2027
14.3.1. Coronary Artery Disease
14.3.2. Peripheral Artery Disease
14.3.3. Neurovascular Disease
14.3.4. Others
14.4. Middle East & Africa Plaque Modification Devices Market Value Forecast, by End-user, 2017–2027
14.4.1. Hospitals
14.4.2. Specialty Clinics
14.4.3. Ambulatory Surgical Centers
14.5. Middle East & Africa Plaque Modification Devices Market Value Forecast, by Country/Sub-region, 2017–2027
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Middle East & Africa Plaque Modification Devices Market Attractiveness Analysis
14.6.1. By Product Type
14.6.2. By Application
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competitive Landscape
15.1. Market Share Analysis, by Company, 2018
15.2. Company Profiles
15.2.1. Boston Scientific Corporation
15.2.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.1.2. Financial Overview
15.2.1.3. Product Portfolio
15.2.1.4. Strategic Overview
15.2.1.5. SWOT Analysis
15.2.2. B. Braun Melsungen
15.2.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.2.2. Financial Overview
15.2.2.3. Product Portfolio
15.2.2.4. Strategic Overview
15.2.2.5. SWOT Analysis
15.2.3. Becton, Dickinson and Company
15.2.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.3.2. Financial Overview
15.2.3.3. Product Portfolio
15.2.3.4. Strategic Overview
15.2.3.5. SWOT Analysis
15.2.4. Cardinal Health
15.2.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.4.2. Financial Overview
15.2.4.3. Product Portfolio
15.2.4.4. Strategic Overview
15.2.4.5. SWOT Analysis
15.2.5. Medtronic
15.2.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.5.2. Financial Overview
15.2.5.3. Product Portfolio
15.2.5.4. Strategic Overview
15.2.5.5. SWOT Analysis
15.2.6. Cardiovascular Systems, Inc.
15.2.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.6.2. Financial Overview
15.2.6.3. Product Portfolio
15.2.6.4. Strategic Overview
15.2.6.5. SWOT Analysis
15.2.7. Microvention, Inc. (Terumo)
15.2.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.7.2. Financial Overview
15.2.7.3. Product Portfolio
15.2.7.4. Strategic Overview
15.2.7.5. SWOT Analysis
15.2.8. Penumbra, Inc.
15.2.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.8.2. Financial Overview
15.2.8.3. Product Portfolio
15.2.8.4. Strategic Overview
15.2.8.5. SWOT Analysis
15.2.9. Biotronik
15.2.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.9.2. Financial Overview
15.2.9.3. Product Portfolio
15.2.9.4. Strategic Overview
15.2.9.5. SWOT Analysis
15.2.10. Stryker
15.2.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.10.2. Financial Overview
15.2.10.3. Product Portfolio
15.2.10.4. Strategic Overview
15.2.10.5. SWOT Analysis
15.2.11. Codman Neuro (Johnson & Johnson)
15.2.11.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.11.2. Financial Overview
15.2.11.3. Product Portfolio
15.2.11.4. Strategic Overview
15.2.11.5. SWOT Analysis
List of Tables
Table 01: Global Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 02: Global Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 03: Global Plaque Modification Devices Market Value (US$ Mn) Forecast, by Application, 2017–2027
Table 04: Global Plaque Modification Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2027
Table 05: Global Plaque Modification Devices Market Value (US$ Mn) Forecast, by Region, 2017–2027
Table 06: North America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 07: North America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 08: North America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Application, 2017–2027
Table 09: North America Plaque Modification Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2027
Table 10: North America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Country, 2017–2027
Table 11: Europe Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 12: Europe Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 13: Europe Plaque Modification Devices Market Value (US$ Mn) Forecast, by Application, 2017–2027
Table 14: Europe Plaque Modification Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2027
Table 15: Europe Plaque Modification Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2027
Table 16: Asia Pacific Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 17: Asia Pacific Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 18: Asia Pacific Plaque Modification Devices Market Value (US$ Mn) Forecast, by Application, 2017–2027
Table 19: Asia Pacific Plaque Modification Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2027
Table 20: Asia Pacific Plaque Modification Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2027
Table 21: Latin America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 22: Latin America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 23: Latin America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Application, 2017–2027
Table 24: Latin America Plaque Modification Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2027
Table 25: Latin America Plaque Modification Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2027
Table 26: Middle East & Africa Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 27: Middle East & Africa Plaque Modification Devices Market Value (US$ Mn) Forecast, by Product Type, 2017–2027
Table 28: Middle East & Africa Plaque Modification Devices Market Value (US$ Mn) Forecast, by Application, 2017–2027
Table 29: Middle East & Africa Plaque Modification Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2027
Table 30: Middle East & Africa Plaque Modification Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2027
List of Figures
Figure 01 : Global Plaque Modification Devices Market Snapshot
Figure 02: Global Plaque Modification Devices Market Value (US$ Mn) and Distribution, by Region, 2018 and 2027
Figure 03: Global Plaque Modification Devices Market Value (US$ Mn) Forecast, 2017?2027
Figure 04: Global Plaque Modification Devices Market Value Share, by Product Type , 2018
Figure 05: Global Plaque Modification Devices Market Value Share, by Application, 2018
Figure 06: Global Plaque Modification Devices Market Value Share, by End-user, 2018
Figure 07: Global Plaque Modification Devices Market Value Share, by Region, 2018
Figure 08: Global Plaque Modification Devices Market Value Share Analysis, by Product Type , 2018 and 2027
Figure 09: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Atherectomy Devices , 2017–2027
Figure 10: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Thrombectomy Devices , 2017–2027
Figure 11: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Chronic Total Occlusion, 2017–2027
Figure 12: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Embolic Protection, 2017–2027
Figure 13: Global Plaque Modification Devices Market Attractiveness Analysis, by Product Type , 2019–2027
Figure 14: Global Plaque Modification Devices Market Value Share Analysis, by Application, 2018 and 2027
Figure 15: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Coronary Disease, 2017–2027
Figure 16: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Peripheral Artery Disease, 2017–2027
Figure 17: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Neurovascular Disease, 2017–2027
Figure 18: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Others, 2017–2027
Figure 19: Global Plaque Modification Devices Market Attractiveness Analysis, by Application, 2019–2027
Figure 20 : Global Plaque Modification Devices Market Value Share Analysis, by End-user, 2018 and 2027
Figure 21: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Hospitals, 2017–2027
Figure 22: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Specialty Clinics, 2017–2027
Figure 23: Global Plaque Modification Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Ambulatory Surgical Centers, 2017–2027
Figure 24: Global Plaque Modification Devices Market Attractiveness Analysis, by End-user, 2019–2027
Figure 25: Global Plaque Modification Devices Market Snapshot, by Country/Sub-region
Figure 26: Global Plaque Modification Devices Market Value Share Analysis, by Region 2018 and 2027
Figure 27: Global Plaque Modification Devices Market Attractiveness Analysis, by Region, 2019–2027
Figure 28: North America Plaque Modification Devices Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, 2017–2027
Figure 29: North America Plaque Modification Devices Market Value Share (%), by Product Type , 2018 and 2027
Figure 30: North America Plaque Modification Devices Market Attractiveness Analysis, by Product Type , 2019–2027
Figure 31: North America Plaque Modification Devices Market Value Share (%), by Application, 2018 and 2027
Figure 32: North America Plaque Modification Devices Market Attractiveness Analysis, by Application, 2019–2027
Figure 33 : North America Plaque Modification Devices Market Value Share (%), by End-user, 2018 and 2027
Figure 34: North America Plaque Modification Devices Market Attractiveness Analysis, by End-user, 2019–2027
Figure 35: North America Plaque Modification Devices Market Value Share (%), by Country, 2018 and 2027
Figure 36: North America Plaque Modification Devices Market Attractiveness Analysis, by Country, 2019–2027
Figure 37: Europe Plaque Modification Devices Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, 2017–2027
Figure 38: Europe Plaque Modification Devices Market Value Share (%), by Product Type , 2018 and 2027
Figure 39: Europe Plaque Modification Devices Market Attractiveness Analysis, by Product Type , 2019–2027
Figure 40: Europe Plaque Modification Devices Market Value Share (%), by Application, 2018 and 2027
Figure 41: Europe Plaque Modification Devices Market Attractiveness Analysis, by Application, 2019–2027
Figure 42: Europe Plaque Modification Devices Market Value Share (%), by End-user, 2018 and 2027
Figure 43: Europe Plaque Modification Devices Market Attractiveness Analysis, by End-user, 2019–2027
Figure 44: Europe Plaque Modification Devices Market Value Share (%), by Country/Sub-region, 2018 and 2027
Figure 45: Europe Plaque Modification Devices Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 46: Asia Pacific Plaque Modification Devices Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, 2017–2027
Figure 47: Asia Pacific Plaque Modification Devices Market Value Share (%), by Product Type , 2018 and 2027
Figure 48: Asia Pacific Plaque Modification Devices Market Attractiveness Analysis, by Product Type , 2019–2027
Figure 49: Asia Pacific Plaque Modification Devices Market Value Share (%), by Application, 2018 and 2027
Figure 50: Asia Pacific Plaque Modification Devices Market Attractiveness Analysis, by Application, 2019–2027
Figure 51: Asia Pacific Plaque Modification Devices Market Value Share (%), by End-user, 2018 and 2027
Figure 52: Asia Pacific Plaque Modification Devices Market Attractiveness Analysis, by End-user, 2019–2027
Figure 53: Asia Pacific Plaque Modification Devices Market Value Share (%), by Country/Sub-region, 2018 and 2027
Figure 54: Asia Pacific Plaque Modification Devices Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 55: Latin America Plaque Modification Devices Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, 2017–2027
Figure 56: Latin America Plaque Modification Devices Market Value Share (%), by Product Type , 2018 and 2027
Figure 57: Latin America Plaque Modification Devices Market Attractiveness Analysis, by Product Type , 2019–2027
Figure 58: Latin America Plaque Modification Devices Market Value Share (%), by Application, 2018 and 2027
Figure 59: Latin America Plaque Modification Devices Market Attractiveness Analysis, by Application, 2019–2027
Figure 60: Latin America Plaque Modification Devices Market Value Share (%), by End-user, 2018 and 2027
Figure 61: Latin America Plaque Modification Devices Market Attractiveness Analysis, by End-user, 2019–2027
Figure 62: Latin America Plaque Modification Devices Market Value Share (%), by Country/Sub-region, 2018 and 2027
Figure 63: Latin America Plaque Modification Devices Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 64: Middle East & Africa Plaque Modification Devices Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, 2017–2027
Figure 65 : Middle East & Africa Plaque Modification Devices Market Value Share (%), by Product Type , 2018 and 2027
Figure 66: Middle East & Africa Plaque Modification Devices Market Attractiveness Analysis, by Product Type , 2019–2027
Figure 67 : Middle East & Africa Plaque Modification Devices Market Value Share (%), by Application, 2018 and 2027
Figure 68: Middle East & Africa Plaque Modification Devices Market Attractiveness Analysis, by Application, 2019–2027
Figure 69 : Middle East & Africa Plaque Modification Devices Market Value Share (%), by End-user, 2018 and 2027
Figure 70: Middle East & Africa Plaque Modification Devices Market Attractiveness Analysis, by End-user, 2019–2027
Figure 71 : Middle East & Africa Plaque Modification Devices Market Value Share (%), by Country/Sub-region, 2018 and 2027
Figure 72: Middle East & Africa Plaque Modification Devices Market Attractiveness Analysis, by Country/Sub-region, 2019–2027