Reports
Reports
Analysts’ Viewpoint
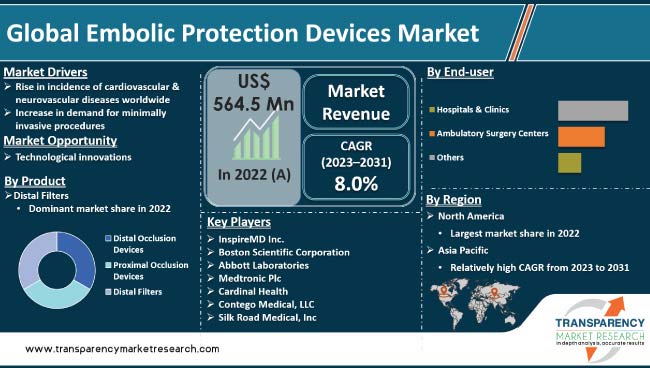
Increase in incidence of cardiovascular & neurovascular diseases across the world and rise in demand for minimally invasive procedures are the major factors driving the global embolic protection devices market. Embolic protection devices are useful in reducing the risk of complications during interventional procedures including angioplasty. Educational initiatives, training programs, and conferences focused on interventional cardiology and neurology have helped disseminate knowledge about the importance of embolic protection devices in preventing complications and improving patient outcomes.
Technological advancements in embolic protection devices have significantly improved their efficacy and safety profiles. Leading manufacturers in the embolic protection devices industry are developing safe, efficient, and innovative devices as well as next-generation devices with enhanced features, such as improved retrieval systems, better filter designs, and advanced imaging capabilities.
Embolic protection device (EPD) has emerged as a significant advancement in interventional cardiology and vascular procedures. Embolic protection devices are specifically designed to minimize the risk of embolization, a condition where debris or clots break loose from a treatment site and travel through the bloodstream, potentially causing blockages and complications in vital organs.
Introduction of embolic protection devices has revolutionized the field of cardiovascular interventions, particularly in complex procedures such as carotid artery stenting and transcatheter aortic valve replacement (TAVR).
These procedures involve the manipulation of diseased vessels and carry a risk of dislodging plaque or debris, leading to embolic events. EPDs provide a valuable solution by capturing and containing these embolic materials, thereby reducing the risk of embolization and related complications.
EPDs typically consist of a filter-based system that is deployed distal to the treatment site. These filters act as a mechanical barrier, catching embolic debris while allowing the passage of blood. After the intervention is completed, the EPD is retrieved, ensuring the captured material is safely removed from the patient's circulation.
Advancements in technology have made embolic protection devices more efficient and user-friendly. Newer devices offer enhanced capture capabilities, ensuring a higher success rate in capturing embolic debris. Moreover, rise in awareness among healthcare professionals about the importance of embolic protection is contributing to the increased demand for these devices.
Cardiovascular & neurovascular diseases are the leading causes of disability and mortality across the globe. Factors such as sedentary lifestyles, poor dietary habits, smoking, and aging population have contributed to rise in prevalence of these conditions. Hence, there is a growing need for advanced medical devices and treatments to address these health issues.
Embolic protection devices are designed to prevent emboli, which are blood clots or other foreign bodies, from traveling to critical organs such as the heart or brain during certain medical procedures. Embolic protection devices act as a barrier, capturing and removing these particles before they can cause damage or block blood flow in vital arteries.
Demand for embolic protection devices is rising due to increase in number of cardiovascular & neurovascular interventions, such as stenting and angioplasty. These procedures carry a risk of dislodging plaque or thrombi, leading to potential stroke or heart attack. By using embolic protection devices, physicians can minimize the risk of such complications, improving patient outcomes.
As technology continues to evolve, embolic protection devices are expected to play an increasingly vital role in cardiovascular interventions, offering enhanced safety, expanded treatment options, and improved patient outcomes.
Minimally invasive procedures offer several advantages over traditional open surgeries, including smaller incisions, reduced pain & scarring, shorter hospital stay, and faster recovery. Hence, more patients and healthcare providers are opting for these procedures across various medical specialties.
People are increasingly seeking alternatives to invasive surgeries that require large incisions and longer recovery period. Minimally invasive procedures offer them the opportunity to undergo treatments with fewer complications, minimal disruption to their daily lives, and quicker return to normal activities. This has fueled the adoption of minimally invasive procedures, which, in turn, is propelling demand for embolic protection devices.
Embolic protection devices play a crucial role in ensuring the safety and effectiveness of minimally invasive procedures. These devices are used during interventions such as transcatheter aortic valve replacement (TAVR), carotid artery stenting, and endovascular aneurysm repair (EVAR), where the risk of embolic events is higher.
By using embolic protection devices, physicians can minimize the risk of emboli dislodgment and subsequent complications, making these procedures safer and more successful. Hence, demand for embolic protection devices is rising significantly with the increase in adoption of minimally invasive procedures.
Manufacturers are developing more advanced and efficient embolic protection devices to meet the growing market needs. Technological advancements are expected to propel the embolic protection devices market progress during the forecast period.
In terms of product, the distal filters segment accounted for the largest global embolic protection devices market share in 2022. This segment is projected to lead the global market during the forecast period.
Distal filters have been widely adopted in various interventional procedures, including percutaneous coronary intervention (PCI) and carotid artery stenting. These devices are designed to capture and prevent embolic debris from reaching distal vessels, thereby reducing the risk of complications such as stroke or organ damage.
High prevalence of cardiovascular diseases, particularly coronary artery disease and carotid artery stenosis, has led to substantial demand for distal filter devices in these specific procedures. Product innovations have further propelled the growth of this segment.
Based on indication, the cardiovascular diseases segment dominated the global market in 2022. Cardiovascular diseases, including coronary artery disease and peripheral artery disease, are among the leading causes of morbidity and mortality worldwide. These conditions often require interventional procedures such as percutaneous coronary intervention (PCI) and angioplasty to restore blood flow to the affected vessels.
During these procedures, embolic protection devices play a crucial role in preventing the release of embolic debris, such as blood clots or plaque fragments, into the bloodstream. By capturing and removing these emboli, these devices help reduce the risk of complications such as myocardial infarction, stroke, or distal vessel occlusion.
The cardiovascular system is highly susceptible to embolic events. The heart and major blood vessels are intricately interconnected, making them vulnerable to embolization from various sources, including dislodged plaque, thrombi, or foreign material. Usage of embolic protection devices provides an added layer of safety by acting as a barrier and preventing embolic debris from reaching critical vessels or organs.
Development of advanced embolic protection devices specifically tailored for cardiovascular procedures has further reinforced their predominant use in this field. These devices are designed to be compatible with the anatomy and physiology of the cardiovascular system, providing optimal protection and ease of use during interventions.
Extensive research and clinical evidence supporting the efficacy and safety of embolic protection devices in cardiovascular procedures have contributed to their widespread adoption.
Based on end-user, the hospitals & clinics segment is projected to account for major share of the global market in the next few years.
Hospitals and clinics are equipped with specialized catheterization laboratories and operating rooms where various cardiovascular and neurovascular interventions are performed. Embolic protection devices are primarily used in these procedures; hence, hospitals & clinics naturally become the primary consumers of these devices.
Large number of patients undergoing interventional procedures for cardiovascular diseases, peripheral artery diseases, and other conditions in hospitals & clinics contributes to the substantial embolic protection devices market demand.
Hospitals and clinics tend to have comprehensive cardiac and vascular care departments, housing specialized healthcare professionals who are familiar with these devices.
Hospitals often have access to the necessary infrastructure and resources to procure and stock medical devices, including embolic protection devices. Hospitals establish relationships with manufacturers and distributors for a steady supply of these devices. The institutional purchasing power of hospitals & clinics allows them to negotiate favorable pricing and procurement contracts, further consolidating their position as major consumers in the market.
According to the latest embolic protection devices market forecast, North America is projected to account for major share of the global industry during the forecast period. The U.S. is the largest contributor to market revenue in the region.
Rise in incidence of cardiovascular & neurovascular diseases and presence of prominent manufacturers are contributing to embolic protection devices market growth in the region.
The embolic protection devices market size in Asia Pacific is projected to be propelled by high prevalence of CVDs and emergence of local companies. Multiple strategic initiatives implemented by innovative companies are anticipated to bolster market expansion in Asia Pacific in the near future.
The latest embolic protection devices market research report provides profiles of leading players operating in the global industry.
InspireMD, Inc., Boston Scientific Corporation, Abbott Laboratories, Medtronic plc, Cardinal Health, Contego Medical, LLC, Silk Road Medical, Inc., Edwards Lifesciences Corporation, and Lepu Medical Technology are the prominent players operating in the global market.
These players are focusing on merger & acquisition, strategic collaborations, and new product launches to expand presence and gain lucrative embolic protection devices industry share. Moreover, these players are following the embolic protection devices market trends to gain revenue benefits.
Each of these players has been profiled in the embolic protection devices market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
|
Size in 2022 |
US$ 564.5 Mn |
|
Forecast (Value) in 2031 |
More than US$ 1.1 Bn |
|
Growth Rate (CAGR) |
8.0% |
|
Forecast Period |
2023-2031 |
|
Historical Data Available for |
2017-2022 |
|
Quantitative Units |
US$ Mn/Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global industry was valued at US$ 564.5 Mn in 2022
It is projected to reach more than US$ 1.1 Bn by 2031
It is expected to advance at a CAGR of 8.0% from 2023 to 2031
The distal filters product segment accounted for the largest share in 2022
North America is the more lucrative region
InspireMD, Inc., Boston Scientific Corporation, Abbott Laboratories, Medtronic plc, Cardinal Health, Contego Medical, LLC, Silk Road Medical, Inc., Edwards Lifesciences Corporation, and Lepu Medical Technology are the prominent players in the market.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Embolic Protection Devices Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Embolic Protection Devices Market Analysis and Forecast, 2020-2031
5. Key Insights
5.1. Technological Advancements
5.2. Disease Prevalence & Incidence Rate Globally With Key Countries
5.3. Regulatory Scenario by Region/Globally
5.4. COVID-19 Impact Analysis
6. Global Embolic Protection Devices Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Product, 2020-2031
6.3.1. Distal Occlusion Devices
6.3.2. Proximal Occlusion Devices
6.3.3. Distal Filters
6.4. Market Attractiveness Analysis, by Product
7. Global Embolic Protection Devices Market Analysis and Forecast, by Application
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Application, 2020-2031
7.3.1. Cardiovascular Diseases
7.3.2. Neurovascular Diseases
7.3.3. Peripheral Vascular Diseases
7.4. Market Attractiveness Analysis, by Application
8. Global Embolic Protection Devices Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by End-user, 2020-2031
8.3.1. Hospitals & Clinics
8.3.2. Ambulatory Surgery Centers
8.3.3. Others
8.4. Market Attractiveness Analysis, by End-user
9. Global Embolic Protection Devices Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region, 2020-2031
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America (LATAM)
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Embolic Protection Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Product, 2020-2031
10.2.1. Distal Occlusion Devices
10.2.2. Proximal Occlusion Devices
10.2.3. Distal Filters
10.3. Market Value Forecast, by Application, 2020-2031
10.3.1. Cardiovascular Diseases
10.3.2. Neurovascular Diseases
10.3.3. Peripheral Vascular Diseases
10.4. Market Value Forecast, by End-user, 2020-2031
10.4.1. Hospitals & Clinics
10.4.2. Ambulatory Surgery Centers
10.4.3. Others
10.5. Market Value Forecast, by Country, 2020-2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Product
10.6.2. By Application
10.6.3. By End-user
10.6.4. By Country
11. Europe Embolic Protection Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Product, 2020-2031
11.2.1. Distal Occlusion Devices
11.2.2. Proximal Occlusion Devices
11.2.3. Distal Filters
11.3. Market Value Forecast, by Application, 2020-2031
11.3.1. Cardiovascular Diseases
11.3.2. Neurovascular Diseases
11.3.3. Peripheral Vascular Diseases
11.4. Market Value Forecast, by End-user, 2020-2031
11.4.1. Hospitals & Clinics
11.4.2. Ambulatory Surgery Centers
11.4.3. Others
11.5. Market Value Forecast, by Country/Sub-region, 2020-2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Italy
11.5.5. Spain
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Product
11.6.2. By Application
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Embolic Protection Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Product, 2020-2031
12.2.1. Distal Occlusion Devices
12.2.2. Proximal Occlusion Devices
12.2.3. Distal Filters
12.3. Market Value Forecast, by Application, 2020-2031
12.3.1. Cardiovascular Diseases
12.3.2. Neurovascular Diseases
12.3.3. Peripheral Vascular Diseases
12.4. Market Value Forecast, by End-user, 2020-2031
12.4.1. Hospitals & Clinics
12.4.2. Ambulatory Surgery Centers
12.4.3. Others
12.5. Market Value Forecast, by Country/Sub-region, 2020-2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Product
12.6.2. By Application
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America (LATAM) Embolic Protection Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Product, 2020-2031
13.2.1. Distal Occlusion Devices
13.2.2. Proximal Occlusion Devices
13.2.3. Distal Filters
13.3. Market Value Forecast, by Application, 2020-2031
13.3.1. Cardiovascular Diseases
13.3.2. Neurovascular Diseases
13.3.3. Peripheral Vascular Diseases
13.4. Market Value Forecast, by End-user, 2020-2031
13.4.1. Hospitals & Clinics
13.4.2. Ambulatory Surgery Centers
13.4.3. Others
13.5. Market Value Forecast, by Country/Sub-region, 2020-2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of LATAM
13.6. Market Attractiveness Analysis
13.6.1. By Product
13.6.2. By Application
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Embolic Protection Devices Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Product, 2020-2031
14.2.1. Distal Occlusion Devices
14.2.2. Proximal Occlusion Devices
14.2.3. Distal Filters
14.3. Market Value Forecast, by Application, 2020-2031
14.3.1. Cardiovascular Diseases
14.3.2. Neurovascular Diseases
14.3.3. Peripheral Vascular Diseases
14.4. Market Value Forecast, by End-user, 2020-2031
14.4.1. Hospitals & Clinics
14.4.2. Ambulatory Surgery Centers
14.4.3. Others
14.5. Market Value Forecast, by Country/Sub-region, 2020-2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of MEA
14.6. Market Attractiveness Analysis
14.6.1. By Product
14.6.2. By Application
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competitive Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company (2022)
15.3. Company Profiles
15.3.1. InspireMD, Inc.
15.3.1.1. Company Overview
15.3.1.2. Product Portfolio
15.3.1.3. SWOT Analysis
15.3.1.4. Financial Overview
15.3.1.5. Strategic Overview
15.3.2. Boston Scientific Corporation
15.3.2.1. Company Overview
15.3.2.2. Product Portfolio
15.3.2.3. SWOT Analysis
15.3.2.4. Financial Overview
15.3.2.5. Strategic Overview
15.3.3. Abbott Laboratories
15.3.3.1. Company Overview
15.3.3.2. Product Portfolio
15.3.3.3. SWOT Analysis
15.3.3.4. Financial Overview
15.3.3.5. Strategic Overview
15.3.4. Medtronic plc
15.3.4.1. Company Overview
15.3.4.2. Product Portfolio
15.3.4.3. SWOT Analysis
15.3.4.4. Financial Overview
15.3.4.5. Strategic Overview
15.3.5. Cardinal Health
15.3.5.1. Company Overview
15.3.5.2. Product Portfolio
15.3.5.3. SWOT Analysis
15.3.5.4. Financial Overview
15.3.5.5. Strategic Overview
15.3.6. Contego Medical, LLC
15.3.6.1. Company Overview
15.3.6.2. Product Portfolio
15.3.6.3. SWOT Analysis
15.3.6.4. Financial Overview
15.3.6.5. Strategic Overview
15.3.7. Silk Road Medical, Inc.
15.3.7.1. Company Overview
15.3.7.2. Product Portfolio
15.3.7.3. SWOT Analysis
15.3.7.4. Financial Overview
15.3.7.5. Strategic Overview
15.3.8. Edwards Lifesciences Corporation
15.3.8.1. Company Overview
15.3.8.2. Product Portfolio
15.3.8.3. SWOT Analysis
15.3.8.4. Financial Overview
15.3.8.5. Strategic Overview
15.3.9. Lepu Medical Technology
15.3.9.1. Company Overview
15.3.9.2. Product Portfolio
15.3.9.3. SWOT Analysis
15.3.9.4. Financial Overview
15.3.9.5. Strategic Overview
List of Tables
Table 01: Global Embolic Protection Devices Market Size (US$ Mn) Forecast, by Product, 2020-2031
Table 02: Global Embolic Protection Devices Market Size (US$ Mn) Forecast, by Application, 2020-2031
Table 03: Global Embolic Protection Devices Market Size (US$ Mn) Forecast, by End-user, 2020-2031
Table 04: Global Embolic Protection Devices Market Size (US$ Mn) Forecast, by Region, 2020-2031
Table 05: North America Embolic Protection Devices Market Size (US$ Mn) Forecast, by Country, 2020-2031
Table 06: North America Embolic Protection Devices Market Size (US$ Mn) Forecast, by Product, 2020-2031
Table 07: North America Embolic Protection Devices Market Size (US$ Mn) Forecast, by Application, 2020-2031
Table 08: North America Embolic Protection Devices Market Size (US$ Mn) Forecast, by End-user, 2020-2031
Table 09: Europe Embolic Protection Devices Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020-2031
Table 10: Europe Embolic Protection Devices Market Size (US$ Mn) Forecast, by Product, 2020-2031
Table 11: Europe Embolic Protection Devices Market Size (US$ Mn) Forecast, by Application, 2020-2031
Table 12: Europe Embolic Protection Devices Market Size (US$ Mn) Forecast, by End-user, 2020-2031
Table 13: Asia Pacific Embolic Protection Devices Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020-2031
Table 14: Asia Pacific Embolic Protection Devices Market Size (US$ Mn) Forecast, by Product, 2020-2031
Table 15: Asia Pacific Embolic Protection Devices Market Size (US$ Mn) Forecast, by Application, 2020-2031
Table 16: Asia Pacific Embolic Protection Devices Market Size (US$ Mn) Forecast, by End-user, 2020-2031
Table 17: Latin America Embolic Protection Devices Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020-2031
Table 18: Latin America Embolic Protection Devices Market Size (US$ Mn) Forecast, by Product, 2020-2031
Table 19: Latin America Embolic Protection Devices Market Size (US$ Mn) Forecast, by Application, 2020-2031
Table 20: Latin America Embolic Protection Devices Market Size (US$ Mn) Forecast, by End-user, 2020-2031
Table 21: Middle East & Africa Embolic Protection Devices Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020-2031
Table 22: Middle East & Africa Embolic Protection Devices Market Size (US$ Mn) Forecast, by Product, 2020-2031
Table 23: Middle East & Africa Embolic Protection Devices Market Size (US$ Mn) Forecast, by Application, 2020-2031
Table 24: Middle East & Africa Embolic Protection Devices Market Size (US$ Mn) Forecast, by End-user, 2020-2031
List of Figures
Figure 01: Global Embolic Protection Devices Market Size (US$ Mn) and Distribution (%), by Region, 2021 and 2031
Figure 02: Global Embolic Protection Devices Market Revenue (US$ Mn), by Product, 2021
Figure 03: Global Embolic Protection Devices Market Value Share, by Product, 2021
Figure 04: Global Embolic Protection Devices Market Revenue (US$ Mn), by Application, 2021
Figure 05: Global Embolic Protection Devices Market Value Share, by Application, 2021
Figure 06: Global Embolic Protection Devices Market Revenue (US$ Mn), by End-user, 2021
Figure 07: Global Embolic Protection Devices Market Value Share, by End-user, 2021
Figure 08: Global Embolic Protection Devices Market Value Share, by Region, 2021
Figure 09: Global Embolic Protection Devices Market Value (US$ Mn) Forecast, 2020-2031
Figure 10: Global Embolic Protection Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 11: Global Embolic Protection Devices Market Attractiveness Analysis, by Product, 2022-2031
Figure 12: Global Embolic Protection Devices Market Value Share Analysis, by Application, 2021 and 2031
Figure 13: Global Embolic Protection Devices Market Attractiveness Analysis, by Application, 2022-2031
Figure 14: Global Embolic Protection Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 15: Global Embolic Protection Devices Market Attractiveness Analysis, by End-user, 2022-2031
Figure 16: Global Embolic Protection Devices Market Value Share Analysis, by Region, 2021 and 2031
Figure 17: Global Embolic Protection Devices Market Attractiveness Analysis, by Region, 2022-2031
Figure 18: North America Embolic Protection Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020-2031
Figure 19: North America Embolic Protection Devices Market Attractiveness Analysis, by Country, 2020-2031
Figure 20: North America Embolic Protection Devices Market Value Share Analysis, by Country, 2021 and 2031
Figure 21: North America Embolic Protection Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 22: North America Embolic Protection Devices Market Value Share Analysis, by Application, 2021 and 2031
Figure 23: North America Embolic Protection Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 24: North America Embolic Protection Devices Market Attractiveness Analysis, by Product, 2022-2031
Figure 25: North America Embolic Protection Devices Market Attractiveness Analysis, by Application, 2022-2031
Figure 26: North America Embolic Protection Devices Market Attractiveness Analysis, by End-user, 2022-2031
Figure 27: Europe Embolic Protection Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020-2031
Figure 28: Europe Embolic Protection Devices Market Attractiveness Analysis, by Country/Sub-region, 2020-2031
Figure 29: Europe Embolic Protection Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 30: Europe Embolic Protection Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 31: Europe Embolic Protection Devices Market Value Share Analysis, by Application, 2021 and 2031
Figure 32: Europe Embolic Protection Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 33: Europe Embolic Protection Devices Market Attractiveness Analysis, by Product, 2022-2031
Figure 34: Europe Embolic Protection Devices Market Attractiveness Analysis, by Application, 2022-2031
Figure 35: Europe Embolic Protection Devices Market Attractiveness Analysis, by End-user, 2022-2031
Figure 36: Asia Pacific Embolic Protection Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020-2031
Figure 37: Asia Pacific Embolic Protection Devices Market Attractiveness Analysis, by Country/Sub-region, 2020-2031
Figure 38: Asia Pacific Embolic Protection Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 39: Asia Pacific Embolic Protection Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 40: Asia Pacific Embolic Protection Devices Market Value Share Analysis, by Application, 2021 and 2031
Figure 41: Asia Pacific Embolic Protection Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 42: Asia Pacific Embolic Protection Devices Market Attractiveness Analysis, by Product, 2022-2031
Figure 43: Asia Pacific Embolic Protection Devices Market Attractiveness Analysis, by Application, 2022-2031
Figure 44: Asia Pacific Embolic Protection Devices Market Attractiveness Analysis, by End-user, 2022-2031
Figure 45: Latin America Embolic Protection Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020-2031
Figure 46: Latin America Embolic Protection Devices Market Attractiveness Analysis, by Country/Sub-region, 2020-2031
Figure 47: Latin America Embolic Protection Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 48: Latin America Embolic Protection Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 49: Latin America Embolic Protection Devices Market Value Share Analysis, by Application, 2021 and 2031
Figure 50: Latin America Embolic Protection Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 51: Latin America Embolic Protection Devices Market Attractiveness Analysis, by Product, 2022-2031
Figure 52: Latin America Embolic Protection Devices Market Attractiveness Analysis, by Application, 2022-2031
Figure 53: Latin America Embolic Protection Devices Market Attractiveness Analysis, by End-user, 2022-2031
Figure 54: Middle East & Africa Embolic Protection Devices Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2020-2031
Figure 55: Middle East & Africa Embolic Protection Devices Market Attractiveness Analysis, by Country/Sub-region, 2020-2031
Figure 56: Middle East & Africa Embolic Protection Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 57: Middle East & Africa Embolic Protection Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 58: Middle East & Africa Embolic Protection Devices Market Value Share Analysis, by Application, 2021 and 2031
Figure 59: Middle East & Africa Embolic Protection Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 60: Middle East & Africa Embolic Protection Devices Market Attractiveness Analysis, by Product, 2022-2031
Figure 61: Middle East & Africa Embolic Protection Devices Market Attractiveness Analysis, by Application, 2022-2031
Figure 62: Middle East & Africa Embolic Protection Devices Market Attractiveness Analysis, by End-user, 2022-2031