Reports
Reports
The monogenetic disease therapy market is witnessing transformative developments due to extensive research being conducted regarding gene-based treatments transitions medicine into a new frontier of therapeutic choices. The expanded utilization of gene-editing technologies such as CRISPR-Cas9, TALENs, and base editors has built-up momentum with respect to developing potential curative options for single-gene disorders. Incentives to invest in research at the global level have also increased, apart from regulatory incentives such as orphan-drug designation and accelerated review for select therapeutics.
Pharmaceutical and biotech companies are forming collaborative partnerships with researchers to help "speed up" the process of transiting therapeutics from academic labs to clinics. The increase in precision diagnostics and the availability of genomic sequencing platforms improve detectability and personalize treatment. Patient advocacy groups are also addressing policy reforms and awareness.
The monogenetic disease treatment market is driven by speedy advancements in gene-editing, high incidence of rare inherited diseases, and national policies in support of the above-mentioned advancements. The merging of high-throughput sequencing and more efficient delivery of gene therapies is facilitating safer and more precise interventions. Healthcare institutions and research institutions are also driven by advancements in asynchronous artificial intelligence.
Continued funding for rare disease programs from public and private sectors is substantially increasing, which supports a clinical pipeline of next generation therapies. Furthermore, continued advancements in the production of viral vectors or cell-based platforms are reducing the cost of production and increasing the scalability of the therapies. Collectively, these innovations lead to strong ecosystem of innovation to pursue curative genetic solutions for lifelong therapies.
| Attribute | Detail |
|---|---|
| Monogenetic Disease Therapy Market Drivers |
|
The development of gene-editing technologies promises to reshape the therapeutic environment for monogenic diseases dramatically. Technologies like CRISPR-Cas9 and base editors can now, in many instances, allow researchers to edit genes with a few associated mutations successfully. These technologies are efficient, reproducible, and therefore appealing regarding the practical application of "clinical" work analysis.
Now, mutagenesis, leading to disease, can be corrected in hematopoietic stem cells, hepatocytes, and retinal cells, and these results can be durable in the clinic. In late 2023, the FDA approved a CRISPR therapy for sickle cell disease, representing the first regulatory approval of in vivo gene-editing therapy anywhere in the world--proving both - safety and efficacy of the therapy. Ultimately, the success of moving technologies from lab investigation to clinical applications has led to hundreds of similar monogenic indications—gene-editing is here to stay as a backbone of future therapeutics.
The increasing identification of single-gene disorders and increasing access to genomic testing worldwide have contributed to the rising demand for monogenic therapies. Genetic conditions can now often be diagnosed in a matter of days due to improvements in next-generation sequencing, which generally leads to earlier intervention and improved outcomes.
Worldwide, governments are ramping up newborn screening to identify newborns’ genetic mutations in the first few days of life in order to start treatment before the child has a lasting effect. Global Genes Alliance estimates that 1 in 10 people on the planet have a rare disease, and almost 80% of those rare diseases are considered genetic, and so the rationale for curative therapies is made evident.
Gene therapy remains at the forefront of treating monogenic diseases as it provides long-term (or even permanent) restoration of defective genes. Additionally, providing sustained expression of therapeutic proteins in patients upon a single administration has entirely changed the landscape of patient care. Advancements in adeno-associated viral (AAV) vectors and lentiviral and non-viral delivery systems have improved treatment safety and specificity.
There has been an increasing regulatory approval due to the ongoing success of patients demonstrating clinical efficacy. For example, Zolgensma has been fast-tracked for approval in spinal muscular atrophy (SMA) showing dramatic survival improvements in infants who otherwise would die within months. This has paved the way for gene therapy to be the backbone for future therapies. With active momentum in research in neurologic, hematologic, and metabolic disease, we are assured that gene therapy is going to remain a focal therapeutic pathway for the next decade.
| Attribute | Detail |
|---|---|
| Leading Region |
|
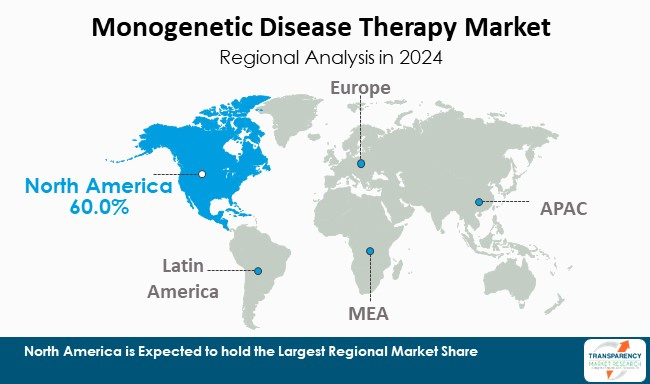
North America leads the monogenetic disease therapy market based on its world-class research infrastructure and active regulatory bodies being in place. The U.S. is especially important for the gene and cell therapy programs, as the FDA has provided several breakthrough and orphan designations to research programs. In addition, the U.S. has a legacy of funding from the National Institutes of Health (NIH) and a number of large academic centers that produce a strong pipeline of early-stage clinical trials and late-phase clinical trials.
More specifically, over 60% of all gene-therapy clinical trials in the world are being undertaken in the U.S.—this is unparalleled. Also, there are specialized hospitals in the U.S. with vector-manufacturing capability and experienced researchers who can help process advanced therapies. In conclusion, these components are essential to make North America the world's leading source of innovation and clinical application for monogenetic disease.
Key players operating in the monogenetic disease therapy industry are investing in innovation, strategic partnerships, and technological advancements. They focus on enhancing imaging clarity and expanding product portfolios, ensuring sustained growth and leadership in the evolving healthcare landscape.
Bayer AG, American Gene Technologies, Adverum Biotechnologies, Inc., CRISPR Therapeutics, Blue Cross Blue Shield Association, MeiraGTx Limited, Sarepta Therapeutics, Inc., Vertex Pharmaceuticals Incorporated, Verve Therapeutics, Inc., Voyager Therapeutics, Inc., Sanofi, Astellas Pharma Inc., Orchard Therapeutics plc, Grifols, S.A., Abeona Therapeutics Inc., Pfizer Inc. are the key players in monogenetic disease therapy market.
Each of these players has been profiled in the monogenetic disease therapy market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
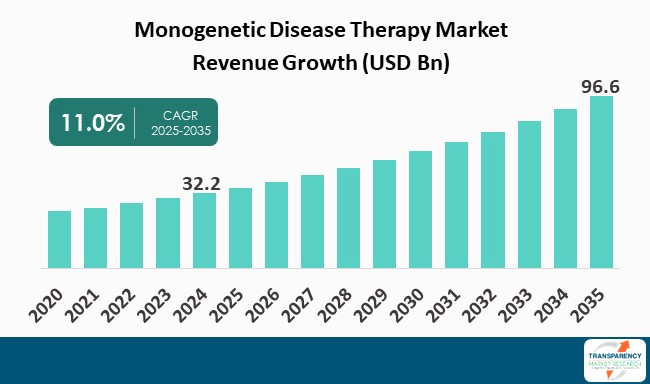
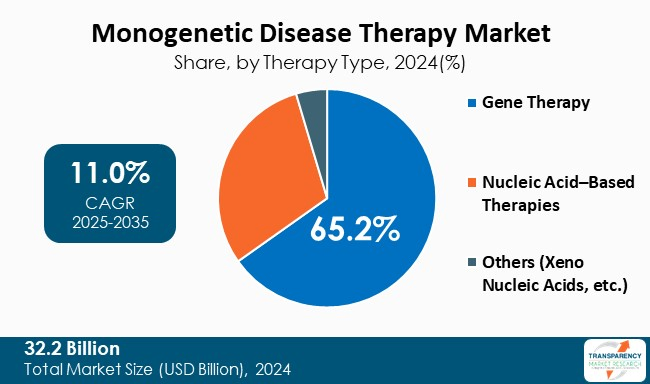
| Size in 2024 | US$ 32.2 Bn |
| Forecast Value in 2035 | US$ 96.6 Bn |
| CAGR | 11.0% |
| Forecast Period | 2025-2035 |
| Historical Data Available for | 2020-2023 |
| Quantitative Units | US$ Bn |
| Monogenetic Disease Therapy Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation | Therapy Type
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
The monogenetic disease therapy market was valued at US$ 32.2 Bn in 2024
The monogenetic disease therapy market is projected to cross US$ 96.6 Bn by the end of 2035
Advancements in gene-editing technologies and rising prevalence of rare genetic disorders
The CAGR is anticipated to be 11.0% from 2025 to 2035
North America is expected to account for the largest share from 2025 to 2035
Bayer AG, American Gene Technologies, Adverum Biotechnologies, Inc., CRISPR Therapeutics, Blue Cross Blue Shield Association, MeiraGTx Limited, Sarepta Therapeutics, Inc., Vertex Pharmaceuticals Incorporated, Verve Therapeutics, Inc., Voyager Therapeutics, Inc., Sanofi, Astellas Pharma Inc., Orchard Therapeutics plc, Grifols, S.A., Abeona Therapeutics Inc., Pfizer Inc., and others
Table 01: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Therapy Type, 2020 to 2035
Table 02: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Gene Therapy, 2020 to 2035
Table 03: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Nucleic Acid-Based Therapies, 2020 to 2035
Table 04: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Age-group, 2020 to 2035
Table 05: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Indication, 2020 to 2035
Table 06: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by End-user, 2020 to 2035
Table 07: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 08: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Therapy Type, 2020 to 2035
Table 09: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Gene Therapy, 2020 to 2035
Table 010: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Nucleic Acid-Based Therapies, 2020 to 2035
Table 11: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Age-group, 2020 to 2035
Table 12: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Indication, 2020 to 2035
Table 13: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by End-user, 2020 to 2035
Table 14: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 15: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Therapy Type, 2020 to 2035
Table 16: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Gene Therapy, 2020 to 2035
Table 17: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Nucleic Acid-Based Therapies, 2020 to 2035
Table 18: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Age-group, 2020 to 2035
Table 19: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Indication, 2020 to 2035
Table 20: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by End-user, 2020 to 2035
Table 21: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 22: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Therapy Type, 2020 to 2035
Table 23: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Gene Therapy, 2020 to 2035
Table 24: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Nucleic Acid-Based Therapies, 2020 to 2035
Table 25: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Age-group, 2020 to 2035
Table 26: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Indication, 2020 to 2035
Table 27: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by End-user, 2020 to 2035
Table 28: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 29: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Therapy Type, 2020 to 2035
Table 30: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Gene Therapy, 2020 to 2035
Table 31: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Nucleic Acid-Based Therapies, 2020 to 2035
Table 32: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Age-group, 2020 to 2035
Table 33: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Indication, 2020 to 2035
Table 34: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by End-user, 2020 to 2035
Table 35: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 36: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Therapy Type, 2020 to 2035
Table 37: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Gene Therapy, 2020 to 2035
Table 38: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Nucleic Acid-Based Therapies, 2020 to 2035
Table 39: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Age-group, 2020 to 2035
Table 40: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Indication, 2020 to 2035
Table 41: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by End-user, 2020 to 2035
Table 42: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Figure 01: Global Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 02: Global Monogenetic Disease Therapy Market Value Share Analysis, by Therapy Type, 2024 and 2035
Figure 03: Global Monogenetic Disease Therapy Market Attractiveness Analysis, by Therapy Type, 2025 to 2035
Figure 04: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Gene Therapy, 2020 to 2035
Figure 05: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Nucleic Acid–Based Therapies, 2020 to 2035
Figure 06: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Others (Transplantation, etc.), 2020 to 2035
Figure 07: Global Monogenetic Disease Therapy Market Value Share Analysis, by Age-group, 2024 and 2035
Figure 08: Global Monogenetic Disease Therapy Market Attractiveness Analysis, by Age-group, 2025 to 2035
Figure 09: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Pediatric, 2020 to 2035
Figure 10: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Adult, 2020 to 2035
Figure 11: Global Monogenetic Disease Therapy Market Value Share Analysis, by Indication, 2024 and 2035
Figure 12: Global Monogenetic Disease Therapy Market Attractiveness Analysis, by Indication, 2025 to 2035
Figure 13: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Sickle Cell Disease, 2020 to 2035
Figure 14: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Cystic Fibrosis, 2020 to 2035
Figure 15: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Polycystic Kidney Disease, 2020 to 2035
Figure 16: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Tay–Sachs Disease, 2020 to 2035
Figure 17: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Others, 2020 to 2035
Figure 18: Global Monogenetic Disease Therapy Market Value Share Analysis, by End-user, 2024 and 2035
Figure 19: Global Monogenetic Disease Therapy Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 20: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Hospitals & Clinics, 2020 to 2035
Figure 21: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Ambulatory Surgical Centers (ASCs), 2020 to 2035
Figure 22: Global Monogenetic Disease Therapy Market Revenue (US$ Bn), by Others (Research Institutes, etc.), 2020 to 2035
Figure 23: Global Monogenetic Disease Therapy Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 24: Global Monogenetic Disease Therapy Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 25: North America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 26: North America Monogenetic Disease Therapy Market Value Share Analysis, by Therapy Type, 2024 and 2035
Figure 27: North America Monogenetic Disease Therapy Market Attractiveness Analysis, by Therapy Type, 2025 to 2035
Figure 28: North America Monogenetic Disease Therapy Market Value Share Analysis, by Age-group, 2024 and 2035
Figure 29: North America Monogenetic Disease Therapy Market Attractiveness Analysis, by Age-group, 2025 to 2035
Figure 30: North America Monogenetic Disease Therapy Market Value Share Analysis, by Indication, 2024 and 2035
Figure 31: North America Monogenetic Disease Therapy Market Attractiveness Analysis, by Indication, 2025 to 2035
Figure 32: North America Monogenetic Disease Therapy Market Value Share Analysis, by End-user, 2024 and 2035
Figure 33: North America Monogenetic Disease Therapy Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 34: North America Monogenetic Disease Therapy Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 35: North America Monogenetic Disease Therapy Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 36: Europe Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 37: Europe Monogenetic Disease Therapy Market Value Share Analysis, by Therapy Type, 2024 and 2035
Figure 38: Europe Monogenetic Disease Therapy Market Attractiveness Analysis, by Therapy Type, 2025 to 2035
Figure 39: Europe Monogenetic Disease Therapy Market Value Share Analysis, by Age-group, 2024 and 2035
Figure 40: Europe Monogenetic Disease Therapy Market Attractiveness Analysis, by Age-group, 2025 to 2035
Figure 41: Europe Monogenetic Disease Therapy Market Value Share Analysis, by Indication, 2024 and 2035
Figure 42: Europe Monogenetic Disease Therapy Market Attractiveness Analysis, by Indication, 2025 to 2035
Figure 43: Europe Monogenetic Disease Therapy Market Value Share Analysis, by End-user, 2024 and 2035
Figure 44: Europe Monogenetic Disease Therapy Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 45: Europe Monogenetic Disease Therapy Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 46: Europe Monogenetic Disease Therapy Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 47: Asia Pacific Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 48: Asia Pacific Monogenetic Disease Therapy Market Value Share Analysis, by Therapy Type, 2024 and 2035
Figure 49: Asia Pacific Monogenetic Disease Therapy Market Attractiveness Analysis, by Therapy Type, 2025 to 2035
Figure 50: Asia Pacific Monogenetic Disease Therapy Market Value Share Analysis, by Age-group, 2024 and 2035
Figure 51: Asia Pacific Monogenetic Disease Therapy Market Attractiveness Analysis, by Age-group, 2025 to 2035
Figure 52: Asia Pacific Monogenetic Disease Therapy Market Value Share Analysis, by Indication, 2024 and 2035
Figure 53: Asia Pacific Monogenetic Disease Therapy Market Attractiveness Analysis, by Indication, 2025 to 2035
Figure 54: Asia Pacific Monogenetic Disease Therapy Market Value Share Analysis, by End-user, 2024 and 2035
Figure 55: Asia Pacific Monogenetic Disease Therapy Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 56: Asia Pacific Monogenetic Disease Therapy Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 57: Asia Pacific Monogenetic Disease Therapy Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 58: Latin America Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 59: Latin America Monogenetic Disease Therapy Market Value Share Analysis, by Therapy Type, 2024 and 2035
Figure 60: Latin America Monogenetic Disease Therapy Market Attractiveness Analysis, by Therapy Type, 2025 to 2035
Figure 61: Latin America Monogenetic Disease Therapy Market Value Share Analysis, by Age-group, 2024 and 2035
Figure 62: Latin America Monogenetic Disease Therapy Market Attractiveness Analysis, by Age-group, 2025 to 2035
Figure 63: Latin America Monogenetic Disease Therapy Market Value Share Analysis, by Indication, 2024 and 2035
Figure 64: Latin America Monogenetic Disease Therapy Market Attractiveness Analysis, by Indication, 2025 to 2035
Figure 65: Latin America Monogenetic Disease Therapy Market Value Share Analysis, by End-user, 2024 and 2035
Figure 66: Latin America Monogenetic Disease Therapy Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 67: Latin America Monogenetic Disease Therapy Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 68: Latin America Monogenetic Disease Therapy Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 69: Middle East and Africa Monogenetic Disease Therapy Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 70: Middle East and Africa Monogenetic Disease Therapy Market Value Share Analysis, by Therapy Type, 2024 and 2035
Figure 71: Middle East and Africa Monogenetic Disease Therapy Market Attractiveness Analysis, by Therapy Type, 2025 to 2035
Figure 72: Middle East and Africa Monogenetic Disease Therapy Market Value Share Analysis, by Age-group, 2024 and 2035
Figure 73: Middle East and Africa Monogenetic Disease Therapy Market Attractiveness Analysis, by Age-group, 2025 to 2035
Figure 74: Middle East and Africa Monogenetic Disease Therapy Market Value Share Analysis, by Indication, 2024 and 2035
Figure 75: Middle East and Africa Monogenetic Disease Therapy Market Attractiveness Analysis, by Indication, 2025 to 2035
Figure 76: Middle East and Africa Monogenetic Disease Therapy Market Value Share Analysis, by End-user, 2024 and 2035
Figure 77: Middle East and Africa Monogenetic Disease Therapy Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 78: Middle East and Africa Monogenetic Disease Therapy Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 79: Middle East and Africa Monogenetic Disease Therapy Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035