Reports
Reports
Analysts’ Viewpoint
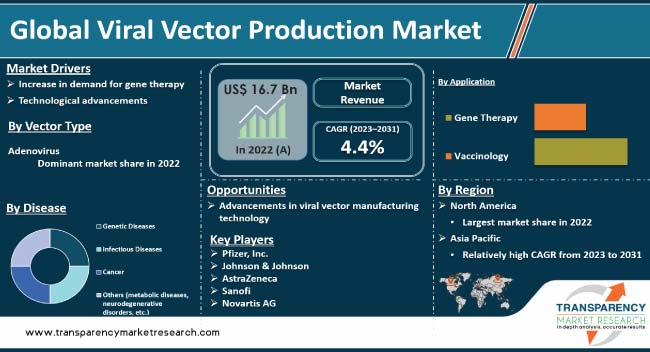
The global viral vector production industry is expected to witness significant growth in the next few years, driven by increase in demand for gene therapy and rise in prevalence of genetic disorders. Development of advanced technologies for viral vector production is expected to propel global viral vector production market growth. Furthermore, increase in demand for gene therapy is likely to accelerate market expansion in the next few years.
Development of advanced technologies for viral vector production is projected to offer lucrative opportunities to market players. Leading players are focusing on development of safe, efficacious, and scalable viral vector production methods in order to increase market share and revenue.
However, high cost of viral vector production and stringent regulatory requirements for gene therapy products are likely to restrain the global market in the next few years.
Viral vector production has been gaining significant attention in the past few years due to its potential applications in gene therapy, vaccine development, and other medical research areas. Viral vectors are modified viruses that are used to deliver genetic material into cells, allowing for the manipulation of gene expression and correction of genetic disorders.
Several types of viral vectors are available in the market, including retroviral vectors, lentiviral vectors, adenoviral vectors, and adeno-associated viral vectors. Each type of vector has its own advantages and disadvantages, depending on the specific application. Retroviral vectors, for example, are commonly used in gene therapy due to their ability to integrate into the host genome, while adenoviral vectors are preferred for vaccine development due to their ability to induce strong immune responses.
Despite their potential benefits, viral vectors pose certain challenges and limitations. One major concern is the risk of immune responses or adverse reactions in patients receiving viral vector-based therapies. Additionally, the production of viral vectors can be complex and expensive, requiring specialized facilities and equipment. Hence, ongoing research and development efforts are focused on improving the safety, efficacy, and scalability of viral vector production methods, with the ultimate goal of bringing these innovative therapies to patients in need.
Gene therapy is a promising treatment option for various genetic disorders, including cancer, inherited diseases, and viral infections. The usage of viral vectors in gene therapy has gained significant attention due to their ability to deliver therapeutic genes into target cells efficiently. Hence, the global viral vector production market is expected to witness robust growth in the next few years. The rise in the prevalence of genetic disorders and chronic diseases, such as cancer, has led to increasing in demand for gene therapies across the world.
According to the World Health Organization (WHO), cancer is one of the leading causes of death globally, with around 9.6 million deaths reported in 2018 alone. Moreover, advancements in biotechnology have enabled researchers to develop new gene therapies that can treat previously untreatable conditions.
Governments of several countries are investing significantly in research & development activities related to gene therapy. For instance, the U.S. Government's National Institutes of Health (NIH) invested over US$ 2 Bn in gene therapy research between 2010 and 2020. Similarly, China's government launched its "Healthy China 2030" initiative aimed at promoting healthcare services and developing innovative treatments such as gene therapies.
Technological advancements have played a crucial role in driving innovation in the global viral vector production market. Significant improvements have been made to enhance efficiency while reducing costs associated with producing high-quality vectors used for various applications such as vaccines or cell-based therapeutics.
CRISPR-Cas9 technology is one major technological advancement that has revolutionized the global market which allows scientists greater precision when editing DNA sequences by cutting out specific sections from genomes without damaging surrounding areas, making it easier to create custom-made viruses tailored specifically toward individual needs.
The development of new viral vector production techniques, such as suspension cell culture systems and transient transfection methods, has enabled researchers to produce high-quality vectors at a lower cost. These advancements have also led to an increase in the scalability of viral vector production, allowing for larger quantities of vectors to be produced in a shorter time.
Additionally, automation technologies have been developed that can streamline the entire process from start to finish. This includes automated bioreactors that can monitor and control various parameters such as temperature, pH levels, and oxygenation rates during cell growth and virus production stages. Such automation not only reduces human error but also increases efficiency while reducing costs associated with manual labor.
In terms of disease, the infectious diseases segment accounted for the largest global viral vector production market share in 2022. This is ascribed to the high prevalence and increase in incidence rates of infectious diseases.
The rise in the need for effective treatments against viral infections is another factor propelling the segment. Viral infections such as HIV/AIDS and hepatitis C are major global health concerns that require effective treatment options. Gene therapy using viral vectors has shown promising results in preclinical studies for treating these diseases.
Another factor contributing to growth in the segment is the development of vaccines against infectious diseases. Vaccines based on viral vectors have been successful in providing protection against various viruses such as influenza and Ebola. These vaccines work by delivering a piece of viral DNA into cells, which then triggers an immune response against that particular virus.
Based on application, the vaccinology segment dominated the global viral vector production market size owing to a wide range of applications, including prevention from life-threatening diseases such as cancer or COVID-19. An increase in demand for new vaccine development due to emerging infectious diseases, such as COVID-19, is a major factor driving the vaccinology segment. Viral vector-based vaccines have emerged as a potential solution due to their ability to induce strong immune responses while being safe. For instance, Oxford-AstraZeneca's COVID-19 vaccine uses an adenoviral vector platform, which has shown an efficacy rate of up to 90% during clinical trials.
Ongoing research activities for developing vaccines against various diseases such as cancer and HIV/AIDS are another factor augmenting the vaccinology segment. Viral vector-based vaccines have shown promising results in preclinical studies for treating these diseases.
In terms of vector type, the adenovirus segment held the largest share of the global market in 2022. This is ascribed to high transduction efficiency and broad host range. Widespread use in gene therapy is one of the major factors bolstering the adenovirus segment. Gene therapy involves introducing genetic material into a patient's cells to treat or prevent disease. Adenoviral vectors are commonly used for this procedure due to their ability to efficiently deliver genes into target cells. Adenoviral vectors have been shown to be safe and effective in clinical trials for various diseases such as cystic fibrosis and hemophilia.
The rise in the usage of adenoviral vectors in vaccine development is also driving the segment. Vaccines based on adenoviral vectors have shown promising results against infectious diseases such as Ebola and Zika viruses. These vaccines work by delivering a piece of viral DNA into cells which then triggers an immune response against that particular virus.
Furthermore, advancements in technology have led to improvements in adenoviral vector production methods. For instance, companies such as Lonza Group AG have developed new viral vector manufacturing processes that allow for large-scale production of high-quality adenoviral vectors at a lower cost.
As per global viral vector production market trends, North America accounted for a major share of the global industry in 2022. The region has a well-established healthcare infrastructure that supports research & development activities related to gene therapy. The U.S. dominated the market in the region owing to the presence of pharmaceutical companies involved in the development of innovative therapies using viral vectors. Furthermore, favorable government initiatives, such as funding support for R&D activities, are propelling the industry in North America.
Asia Pacific is projected to be the fastest-growing region during the forecast period. This can be ascribed to factors such as an increase in disposable income, a rise in awareness about advanced therapies such as gene therapy & cell-based immunotherapy, and a surge in investments by key players in emerging economies such as China and India. Furthermore, countries such as Japan are contributing to the growth of the market in the region due to technological advancements and a supportive regulatory environment. Several local players are entering into partnerships with international firms or acquiring them, which will help them expand their product portfolio while offering cost-effective solutions.
The report includes vital information about the key companies operating in the viral vector production market. Companies focus on strategies such as product launches, divestiture, mergers & acquisitions (M&A), and partnerships to strengthen their position in the market. Pfizer, Inc., Johnson & Johnson, AstraZeneca, Sanofi, Novartis AG, Spark Therapeutics, Inc. (F. Hoffmann-La Roche Ltd.), GlaxoSmithKline plc, Merck KGaA (Merck & Co., Inc.), Lonza, FUJIFILM Diosynth Biotechnologies, Inc., Oxford Biomedica plc, Amgen, Inc., Ferring B.V., uniQure N.V., bluebird bio, Inc., GenScript ProBio, Thermo Fisher Scientific, Inc., and Gamaleya Research Institute Industries are prominent players operating in the market.
Each of these players in the market has been profiled in the report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Size Value in 2022 |
US$ 16.7 Bn |
|
Forecast (Value) in 2031 |
More than US$ 17.3 Bn |
|
Compound Annual Growth Rate (CAGR) |
4.4% |
|
Forecast Period |
2023-2031 |
|
Historical Data Available for |
2017-2021 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global industry was valued at US$ 16.7 Bn in 2022
It is projected to reach more than US$ 17.3 Bn by 2031
The market is anticipated to grow at a CAGR of 4.4% from 2023 to 2031
Increase in demand for gene therapy and technological advancements are driving the global market
The infectious diseases segment held the largest market share in 2022
North America is expected to account for major share of the global market during the forecast period
Pfizer, Inc., Johnson & Johnson, AstraZeneca, Sanofi, Novartis AG, Spark Therapeutics, Inc. (F. Hoffmann-La Roche Ltd.), GlaxoSmithKline plc, Merck KGaA (Merck & Co., Inc.), Lonza, FUJIFILM Diosynth Biotechnologies, Inc., Oxford Biomedica plc, Amgen, Inc., Ferring B.V., uniQure N.V., bluebird bio, Inc., GenScript ProBio, Thermo Fisher Scientific, Inc., and Gamaleya Research Institute are the prominent players in the market.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Viral Vector Production Market
4. Market Overview
4.1. Introduction
4.1.1. Vector Type Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Viral Vector Production Market Analysis and Forecast, 2017-2031
5. Key Insights
5.1. Evolution of Gene Therapy
5.2. Pipeline Analysis
5.3. Regulatory Scenario, by Region/Globally
5.4. COVID-19 Pandemic Impact on Industry (value chain and short / mid / long term impact)
6. Global Viral Vector Production Market Analysis and Forecast, by Vector Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Vector Type, 2017-2031
6.3.1. Adenovirus
6.3.2. Adeno-associated virus
6.3.3. Retroviruses
6.3.4. Others (baculoviruses, lentivirus, etc.)
6.4. Market Attractiveness Analysis, by Vector Type
7. Global Viral Vector Production Market Analysis and Forecast, by Disease
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Disease, 2017-2031
7.3.1. Genetic Diseases
7.3.2. Infectious Diseases
7.3.3. Cancer
7.3.4. Others (metabolic diseases, neurodegenerative disorders, etc.)
7.4. Market Attractiveness Analysis, by Disease
8. Global Viral Vector Production Market Analysis and Forecast, by Application
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Application, 2017-2031
8.3.1. Gene Therapy
8.3.2. Vaccinology
8.4. Market Attractiveness Analysis, by Application
9. Global Viral Vector Production Market Analysis and Forecast, by Mode
9.1. Introduction & Definition
9.2. Key Findings / Developments
9.3. Market Value Forecast, by Mode, 2017-2031
9.3.1. Transient Transfection
9.3.2. Stable Producer Cell Lines
9.4. Market Attractiveness Analysis, by Mode
10. Global Viral Vector Production Market Analysis and Forecast, by End-user
10.1. Introduction & Definition
10.2. Key Findings / Developments
10.3. Market Value Forecast, by End-user, 2017-2031
10.3.1. Pharmaceutical & Biotechnology Companies
10.3.2. Academic & Research Institutes
10.3.3. CROs & CDMOs
10.4. Market Attractiveness Analysis, by End-user
11. Global Viral Vector Production Market Analysis and Forecast, by Region
11.1. Key Findings
11.2. Market Value Forecast, by Region
11.2.1. North America
11.2.2. Europe
11.2.3. Asia Pacific
11.2.4. Latin America
11.2.5. Middle East & Africa
11.3. Market Attractiveness Analysis, by Region
12. North America Viral Vector Production Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Vector Type, 2017-2031
12.2.1. Adenovirus
12.2.2. Adeno-associated virus
12.2.3. Retroviruses
12.2.4. Others (baculoviruses, lentivirus, etc.)
12.3. Market Value Forecast, by Disease, 2017-2031
12.3.1. Genetic Diseases
12.3.2. Infectious Diseases
12.3.3. Cancer
12.3.4. Others (metabolic diseases, neurodegenerative disorders, etc.)
12.4. Market Value Forecast, by Application, 2017-2031
12.4.1. Gene Therapy
12.4.2. Vaccinology
12.5. Market Value Forecast, by Mode, 2017-2031
12.5.1. Transient Transfection
12.5.2. Stable Producer Cell Lines
12.6. Market Value Forecast, by End-user, 2017-2031
12.6.1. Pharmaceutical & Biotechnology Companies
12.6.2. Academic & Research Institutes
12.6.3. CROs & CDMOs
12.7. Market Value Forecast, by Country, 2017-2031
12.7.1. U.S.
12.7.2. Canada
12.8. Market Attractiveness Analysis
12.8.1. By Vector Type
12.8.2. By Disease
12.8.3. By Application
12.8.4. By Mode
12.8.5. By End-user
12.8.6. By Country
13. Europe Viral Vector Production Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Vector Type, 2017-2031
13.2.1. Adenovirus
13.2.2. Adeno-associated virus
13.2.3. Retroviruses
13.2.4. Others (baculoviruses, lentivirus, etc.)
13.3. Market Value Forecast, by Disease, 2017-2031
13.3.1. Genetic Diseases
13.3.2. Infectious Diseases
13.3.3. Cancer
13.3.4. Others (metabolic diseases, neurodegenerative disorders, etc.)
13.4. Market Value Forecast, by Application, 2017-2031
13.4.1. Gene Therapy
13.4.2. Vaccinology
13.5. Market Value Forecast, by Mode, 2017-2031
13.5.1. Transient Transfection
13.5.2. Stable Producer Cell Lines
13.6. Market Value Forecast, by End-user, 2017-2031
13.6.1. Pharmaceutical & Biotechnology Companies
13.6.2. Academic & Research Institutes
13.6.3. CROs & CDMOs
13.7. Market Value Forecast, by Country/Sub-region, 2017-2031
13.7.1. Germany
13.7.2. U.K.
13.7.3. France
13.7.4. Spain
13.7.5. Italy
13.7.6. Rest of Europe
13.8. Market Attractiveness Analysis
13.8.1. By Vector Type
13.8.2. By Disease
13.8.3. By Application
13.8.4. By Mode
13.8.5. By End-user
13.8.6. By Country/Sub-region
14. Asia Pacific Viral Vector Production Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Vector Type, 2017-2031
14.2.1. Adenovirus
14.2.2. Adeno-associated virus
14.2.3. Retroviruses
14.2.4. Others (baculoviruses, lentivirus, etc.)
14.3. Market Value Forecast, by Diseases, 2017-2031
14.3.1. Genetic Diseases
14.3.2. Infectious Diseases
14.3.3. Cancer
14.3.4. Others (metabolic diseases, neurodegenerative disorders, etc.)
14.4. Market Value Forecast, by Application, 2017-2031
14.4.1. Gene Therapy
14.4.2. Vaccinology
14.5. Market Value Forecast, by Mode, 2017-2031
14.5.1. Transient Transfection
14.5.2. Stable Producer Cell Lines
14.6. Market Value Forecast, by End-user, 2017-2031
14.6.1. Pharmaceutical & Biotechnology Companies
14.6.2. Academic & Research Institutes
14.6.3. CROs & CDMOs
14.7. Market Value Forecast, by Country/Sub-region, 2017-2031
14.7.1. China
14.7.2. Japan
14.7.3. India
14.7.4. Australia & New Zealand
14.7.5. Rest of Asia Pacific
14.8. Market Attractiveness Analysis
14.8.1. By Vector Type
14.8.2. By Disease
14.8.3. By Application
14.8.4. By Mode
14.8.5. By End-user
14.8.6. By Country/Sub-region
15. Latin America Viral Vector Production Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Vector Type, 2017-2031
15.2.1. Adenovirus
15.2.2. Adeno-associated virus
15.2.3. Retroviruses
15.2.4. Others (baculoviruses, lentivirus, etc.)
15.3. Market Value Forecast, by Disease, 2017-2031
15.3.1. Genetic Diseases
15.3.2. Infectious Diseases
15.3.3. Cancer
15.3.4. Others (metabolic diseases, neurodegenerative disorders, etc.)
15.4. Market Value Forecast, by Application, 2017-2031
15.4.1. Gene Therapy
15.4.2. Vaccinology
15.5. Market Value Forecast, by Mode, 2017-2031
15.5.1. Transient Transfection
15.5.2. Stable Producer Cell Lines
15.6. Market Value Forecast, by End-user, 2017-2031
15.6.1. Pharmaceutical & Biotechnology Companies
15.6.2. Academic & Research Institutes
15.6.3. CROs & CDMOs
15.7. Market Value Forecast, by Country/Sub-region, 2017-2031
15.7.1. Brazil
15.7.2. Mexico
15.7.3. Rest of Latin America
15.8. Market Attractiveness Analysis
15.8.1. By Vector Type
15.8.2. By Disease
15.8.3. By Application
15.8.4. By Mode
15.8.5. By End-user
15.8.6. By Country/Sub-region
16. Middle East & Africa Viral Vector Production Market Analysis and Forecast
16.1. Introduction
16.1.1. Key Findings
16.2. Market Value Forecast, by Vector Type, 2017-2031
16.2.1. Adenovirus
16.2.2. Adeno-associated virus
16.2.3. Retroviruses
16.2.4. Others (baculoviruses, lentivirus, etc.)
16.3. Market Value Forecast, by Disease, 2017-2031
16.3.1. Genetic Diseases
16.3.2. Infectious Diseases
16.3.3. Cancer
16.3.4. Others (metabolic diseases, neurodegenerative disorders, etc.)
16.4. Market Value Forecast, by Application, 2017-2031
16.4.1. Gene Therapy
16.4.2. Vaccinology
16.5. Market Value Forecast, by Mode, 2017-2031
16.5.1. Transient Transfection
16.5.2. Stable Producer Cell Lines
16.6. Market Value Forecast, by End-user, 2017-2031
16.6.1. Pharmaceutical & Biotechnology Companies
16.6.2. Academic & Research Institutes
16.6.3. CROs & CDMOs
16.7. Market Value Forecast, by Country/Sub-region, 2017-2031
16.7.1. GCC Countries
16.7.2. South Africa
16.7.3. Rest of Middle East & Africa
16.8. Market Attractiveness Analysis
16.8.1. By Vector Type
16.8.2. By Disease
16.8.3. By Application
16.8.4. By Mode
16.8.5. By End-user
16.8.6. By Country/Sub-region
17. Competition Landscape
17.1. Market Player - Competition Matrix (By Tier and Size of companies)
17.2. Market Share Analysis By Company (2021)
17.3. Company Profiles
17.3.1. Pfizer, Inc.
17.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.1.2. Test Type Portfolio
17.3.1.3. Financial Overview
17.3.1.4. SWOT Analysis
17.3.1.5. Strategic Overview
17.3.2. Johnson & Johnson
17.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.2.2. Test Type Portfolio
17.3.2.3. Financial Overview
17.3.2.4. SWOT Analysis
17.3.2.5. Strategic Overview
17.3.3. AstraZeneca
17.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.3.2. Test Type Portfolio
17.3.3.3. Financial Overview
17.3.3.4. SWOT Analysis
17.3.3.5. Strategic Overview
17.3.4. Sanofi
17.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.4.2. Test Type Portfolio
17.3.4.3. Financial Overview
17.3.4.4. SWOT Analysis
17.3.4.5. Strategic Overview
17.3.5. Novartis AG
17.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.5.2. Test Type Portfolio
17.3.5.3. Financial Overview
17.3.5.4. SWOT Analysis
17.3.5.5. Strategic Overview
17.3.6. Spark Therapeutics, Inc. (F. Hoffmann-La Roche Ltd.)
17.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.6.2. Test Type Portfolio
17.3.6.3. Financial Overview
17.3.6.4. SWOT Analysis
17.3.6.5. Strategic Overview
17.3.7. GlaxoSmithKline plc
17.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.7.2. Test Type Portfolio
17.3.7.3. Financial Overview
17.3.7.4. SWOT Analysis
17.3.7.5. Strategic Overview
17.3.8. Merck KGaA (Merck & Co., Inc.)
17.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.8.2. Test Type Portfolio
17.3.8.3. Financial Overview
17.3.8.4. SWOT Analysis
17.3.8.5. Strategic Overview
17.3.9. Lonza
17.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.9.2. Test Type Portfolio
17.3.9.3. Financial Overview
17.3.9.4. SWOT Analysis
17.3.9.5. Strategic Overview
17.3.10. FUJIFILM Diosynth Biotechnologies Inc.
17.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.10.2. Test Type Portfolio
17.3.10.3. Financial Overview
17.3.10.4. SWOT Analysis
17.3.10.5. Strategic Overview
17.3.11. Oxford Biomedica plc
17.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.11.2. Test Type Portfolio
17.3.11.3. Financial Overview
17.3.11.4. SWOT Analysis
17.3.11.5. Strategic Overview
17.3.12. Amgen Inc.
17.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.12.2. Test Type Portfolio
17.3.12.3. Financial Overview
17.3.12.4. SWOT Analysis
17.3.12.5. Strategic Overview
17.3.13. Ferring B.V.
17.3.13.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.13.2. Test Type Portfolio
17.3.13.3. Financial Overview
17.3.13.4. SWOT Analysis
17.3.13.5. Strategic Overview
17.3.14. uniQure N.V.
17.3.14.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.14.2. Test Type Portfolio
17.3.14.3. Financial Overview
17.3.14.4. SWOT Analysis
17.3.14.5. Strategic Overview
17.3.15. bluebird bio, Inc.
17.3.15.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.15.2. Test Type Portfolio
17.3.15.3. Financial Overview
17.3.15.4. SWOT Analysis
17.3.15.5. Strategic Overview
17.3.16. GenScript ProBio
17.3.16.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.16.2. Test Type Portfolio
17.3.16.3. Financial Overview
17.3.16.4. SWOT Analysis
17.3.16.5. Strategic Overview
17.3.17. Thermo Fisher Scientific, Inc.
17.3.17.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.17.2. Test Type Portfolio
17.3.17.3. Financial Overview
17.3.17.4. SWOT Analysis
17.3.17.5. Strategic Overview
17.3.18. Gamaleya Research Institute
17.3.18.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.18.2. Test Type Portfolio
17.3.18.3. Financial Overview
17.3.18.4. SWOT Analysis
17.3.18.5. Strategic Overview
List of Tables
Table 01: Global Viral Vector Production Market Value (US$ Mn) Forecast, by Vector Type, 2017-2032
Table 02: Global Viral Vector Production Market Value (US$ Mn) Forecast, by Disease, 2017-2032
Table 03: Global Viral Vector Production Market Value (US$ Mn) Forecast, by Application, 2017-2032
Table 04: Global Viral Vector Production Market Value (US$ Mn) Forecast, by Mode, 2017-2032
Table 05: Global Viral Vector Production Market Value (US$ Mn) Forecast, by End-user, 2017-2032
Table 06: Global Viral Vector Production Market Value (US$ Mn) Forecast, by Region, 2017-2032
Table 07: North America Viral Vector Production Market Value (US$ Mn) Forecast, by Country, 2017-2032
Table 08: North America Viral Vector Production Market Value (US$ Mn) Forecast, by Vector Type, 2017-2032
Table 09: North America Viral Vector Production Market Value (US$ Mn) Forecast, by Disease, 2017-2032
Table 10: North America Viral Vector Production Market Value (US$ Mn) Forecast, by Application, 2017-2032
Table 11: North America Viral Vector Production Market Value (US$ Mn) Forecast, by Mode, 2017-2032
Table 12: North America Viral Vector Production Market Value (US$ Mn) Forecast, by End-user, 2017-2032
Table 13: Europe Viral Vector Production Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2032
Table 14: Europe Viral Vector Production Market Value (US$ Mn) Forecast, by Vector Type, 2017-2032
Table 15: Europe Viral Vector Production Market Value (US$ Mn) Forecast, by Disease, 2017-2032
Table 16: Europe Viral Vector Production Market Value (US$ Mn) Forecast, by Application, 2017-2032
Table 17: Europe Viral Vector Production Market Value (US$ Mn) Forecast, by Mode, 2017-2032
Table 18: Europe Viral Vector Production Market Value (US$ Mn) Forecast, by End-user, 2017-2032
Table 19: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2032
Table 20: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, by Vector Type, 2017-2032
Table 21: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, by Disease, 2017-2032
Table 22: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, by Application, 2017-2032
Table 23: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, by Mode, 2017-2032
Table 24: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, by End-user, 2017-2032
Table 25: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2032
Table 26: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, by Vector Type, 2017-2032
Table 27: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, by Disease, 2017-2032
Table 28: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, by Application, 2017-2032
Table 29: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, by Mode, 2017-2032
Table 30: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, by End-user, 2017-2032
Table 31: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2032
Table 32: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, by Vector Type, 2017-2032
Table 33: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, by Disease, 2017-2032
Table 34: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, by Application, 2017-2032
Table 35: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, by Mode, 2017-2032
Table 36: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, by End-user, 2017-2032
List of Figures
Figure 01: Global Viral Vector Production Market Value (US$ Mn) Forecast, 2017-2032
Figure 02: Global Viral Vector Production Market Value Share, by Vector Type, 2022
Figure 03: Global Viral Vector Production Market Value Share, by Disease, 2022
Figure 04: Global Viral Vector Production Market Value Share, by Application, 2022
Figure 05: Global Viral Vector Production Market Value Share, by Mode, 2022
Figure 06: Global Viral Vector Production Market Value Share, by End-user, 2022
Figure 07: Global Viral Vector Production Market Value Share, by Region, 2022
Figure 08: Global Viral Vector Production Market Value Share Analysis, by Vector Type, 2022 and 2032
Figure 09: Global Viral Vector Production Market Attractiveness Analysis, by Vector Type, 2023-2031
Figure 10: Global Viral Vector Production Market (US$ Mn), by Adenovirus, 2017-2032
Figure 11 Global Viral Vector Production Market (US$ Mn), by Adeno-associated Virus, 2017-2031
Figure 12: Global Viral Vector Production Market (US$ Mn), by Retroviruses, 2017-2032
Figure 13: Global Viral Vector Production Market (US$ Mn), by Others, 2017-2032
Figure 14: Global Viral Vector Production Market Value Share Analysis, by Disease, 2022 and 2032
Figure 15: Global Viral Vector Production Market Attractiveness Analysis, by Disease, 2023-2031
Figure 16: Global Viral Vector Production Market (US$ Mn), by Genetic Diseases, 2017-2032
Figure 17: Global Viral Vector Production Market (US$ Mn), by Infectious Diseases, 2017-2032
Figure 18: Global Viral Vector Production Market (US$ Mn), by Cancer, 2017-2032
Figure 19: Global Viral Vector Production Market (US$ Mn), by Others, 2017-2032
Figure 20: Global Viral Vector Production Market Value Share Analysis, by Application, 2022 and 2032
Figure 21: Global Viral Vector Production Market Attractiveness Analysis, by Application, 2023-2032
Figure 22: Global Viral Vector Production Market (US$ Mn), by Gene therapy, 2017-2032
Figure 23: Global Viral Vector Production Market (US$ Mn), by Vaccinology, 2017-2032
Figure 24: Global Viral Vector Production Market Value Share Analysis, by Mode, 2022 and 2032
Figure 25: Global Viral Vector Production Market Attractiveness Analysis, by Mode, 2023-2032
Figure 26: Global Viral Vector Production Market (US$ Mn), by Transient Transfection, 2017-2032
Figure 27: Global Viral Vector Production Market (US$ Mn), by Stable Producer Cell Lines, 2017-2032
Figure 28: Global Viral Vector Production Market Value Share Analysis, by End-user, 2022 and 2032
Figure 29: Global Viral Vector Production Market Attractiveness Analysis, by End-user, 2023-2032
Figure 30: Global Viral Vector Production Market (US$ Mn), by Pharmaceutical & Biotechnology Companies, 2017-2032
Figure 31: Global Viral Vector Production Market (US$ Mn), by Academic & Research Institutes, 2017-2032
Figure 32: Global Viral Vector Production Market Value Share Analysis, by Region, 2022 and 2032
Figure 33: Global Viral Vector Production Market Attractiveness Analysis, by Region, 2023-2032
Figure 34: North America Viral Vector Production Market Value (US$ Mn) Forecast, 2017-2032
Figure 35: North America Viral Vector Production Market Value Share Analysis, by Country, 2022 and 2032
Figure 36: North America Viral Vector Production Market Attractiveness Analysis, by Country, 2023-2032
Figure 37: North America Viral Vector Production Market Value Share Analysis, by Vector Type, 2022 and 2032
Figure 38: North America Viral Vector Production Market Attractiveness Analysis, by Vector Type, 2023-2032
Figure 39: North America Viral Vector Production Market Value Share Analysis, by Disease, 2022 and 2032
Figure 40: North America Viral Vector Production Market Attractiveness Analysis, by Disease, 2023-2032
Figure 41: North America Viral Vector Production Market Value Share Analysis, by Application, 2022 and 2032
Figure 42: North America Viral Vector Production Market Attractiveness Analysis, by Application, 2023-2032
Figure 43: North America Viral Vector Production Market Value Share Analysis, by Mode, 2022 and 2032
Figure 44: North America Viral Vector Production Market Attractiveness Analysis, by Mode, 2023-2032
Figure 45: North America Viral Vector Production Market Value Share Analysis, by End-user, 2022 and 2032
Figure 46: North America Viral Vector Production Market Attractiveness Analysis, by End-user, 2023-2032
Figure 47: Europe Viral Vector Production Market Value (US$ Mn) Forecast, 2017-2032
Figure 48: Europe Viral Vector Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2032
Figure 49: Europe Viral Vector Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2032
Figure 50: Europe Viral Vector Production Market Value Share Analysis, by Vector Type, 2022 and 2032
Figure 51: Europe Viral Vector Production Market Attractiveness Analysis, by Vector Type, 2023-2032
Figure 52: Europe Viral Vector Production Market Value Share Analysis, by Disease, 2022 and 2032
Figure 53: Europe Viral Vector Production Market Attractiveness Analysis, by Disease, 2023-2032
Figure 54: Europe Viral Vector Production Market Value Share Analysis, by Application, 2022 and 2032
Figure 55: Europe Viral Vector Production Market Attractiveness Analysis, by Application, 2023-2032
Figure 56: Europe Viral Vector Production Market Value Share Analysis, by Mode, 2022 and 2032
Figure 57: Europe Viral Vector Production Market Attractiveness Analysis, by Mode, 2023-2032
Figure 58: Europe Viral Vector Production Market Value Share Analysis, by End-user, 2022 and 2032
Figure 59: Europe Viral Vector Production Market Attractiveness Analysis, by End-user, 2023-2032
Figure 60: Asia Pacific Viral Vector Production Market Value (US$ Mn) Forecast, 2017-2032
Figure 61: Asia Pacific Viral Vector Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2032
Figure 62: Asia Pacific Viral Vector Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2032
Figure 63: Asia Pacific Viral Vector Production Market Value Share Analysis, by Vector Type, 2022 and 2032
Figure 64: Asia Pacific Viral Vector Production Market Attractiveness Analysis, by Vector Type, 2023-032
Figure 65: Asia Pacific Viral Vector Production Market Value Share Analysis, by Disease, 2022 and 2032
Figure 66: Asia Pacific Viral Vector Production Market Attractiveness Analysis, by Disease, 2023-2032
Figure 67: Asia Pacific Viral Vector Production Market Value Share Analysis, by Application, 2022 and 2032
Figure 68: Asia Pacific Viral Vector Production Market Attractiveness Analysis, by Application, 2023-2032
Figure 69: Asia Pacific Viral Vector Production Market Value Share Analysis, by Mode, 2022 and 2032
Figure 70: Asia Pacific Viral Vector Production Market Attractiveness Analysis, by Mode, 2023-2032
Figure 71: Asia Pacific Viral Vector Production Market Value Share Analysis, by End-user, 2022 and 2032
Figure 72: Asia Pacific Viral Vector Production Market Attractiveness Analysis, by End-user, 2023-2032
Figure 73: Latin America Viral Vector Production Market Value (US$ Mn) Forecast, 2017-2032
Figure 74: Latin America Viral Vector Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2032
Figure 75: Latin America Viral Vector Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2032
Figure 76: Latin America Viral Vector Production Market Value Share Analysis, by Vector Type, 2022 and 2032
Figure 77: Latin America Viral Vector Production Market Attractiveness Analysis, by Vector Type, 2023-2032
Figure 78: Latin America Viral Vector Production Market Value Share Analysis, by Disease, 2022 and 2032
Figure 79: Latin America Viral Vector Production Market Attractiveness Analysis, by Disease, 2023-2032
Figure 80: Latin America Viral Vector Production Market Value Share Analysis, by Application, 2022 and 2032
Figure 81: Latin America Viral Vector Production Market Attractiveness Analysis, by Application, 2023-2032
Figure 82: Latin America Viral Vector Production Market Value Share Analysis, by Mode, 2022 and 2032
Figure 83: Latin America Viral Vector Production Market Attractiveness Analysis, by Mode, 2023-2032
Figure 84: Latin America Viral Vector Production Market Value Share Analysis, by End-user, 2022 and 2032
Figure 85: Latin America Viral Vector Production Market Attractiveness Analysis, by End-user, 2023-2032
Figure 86: Middle East & Africa Viral Vector Production Market Value (US$ Mn) Forecast, 2017-2032
Figure 87: Middle East & Africa Viral Vector Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2032
Figure 88: Middle East & Africa Viral Vector Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2032
Figure 89: Middle East & Africa Viral Vector Production Market Value Share Analysis, by Vector Type, 2022 and 2032
Figure 90: Middle East & Africa Viral Vector Production Market Attractiveness Analysis, by Vector Type, 2023-2032
Figure 91: Middle East & Africa Viral Vector Production Market Value Share Analysis, by Disease, 2022 and 2032
Figure 92: Middle East & Africa Viral Vector Production Market Attractiveness Analysis, by Disease, 2023-2032
Figure 93: Middle East & Africa Viral Vector Production Market Value Share Analysis, by Application, 2022 and 2032
Figure 94: Middle East & Africa Viral Vector Production Market Attractiveness Analysis, by Application, 2023-2032
Figure 95: Middle East & Africa Viral Vector Production Market Value Share Analysis, by Mode, 2022 and 2032
Figure 96: Middle East & Africa Viral Vector Production Market Attractiveness Analysis, by Mode, 2023-2032
Figure 97: Middle East & Africa Viral Vector Production Market Value Share Analysis, by End-user, 2022 and 2032
Figure 98: Middle East & Africa Viral Vector Production Market Attractiveness Analysis, by End-user, 2023-2032
Figure 99: Global Viral Vector Production Market Share, by Company, 2022