Reports
Reports
Analysts’ Viewpoint on Thoracic Drainage Devices Market Scenario
At the beginning of the 21st century, cardiothoracic surgery was arguably the most successful of all medical specialties. Emerging economies, including China, India, South Korea, Brazil, Russia, South Africa, and Turkey, provide significant opportunities for prominent companies in the thoracic drainage devices market owing to their massive population, specifically in China and India. However, the price factor is a concern in the economies mentioned above.
The COVID-19 pandemic has presented considerable opportunities in the global thoracic drainage devices market. Demand for chest wall drainage devices or thoracic drainage devices is increasing due to the surge in incidence of respiratory diseases and cardiovascular diseases during the pandemic. Key players operating in the thoracic drainage devices market should focus on continued investment in advancements in thoracic drainage devices to introduce innovative products in the market.
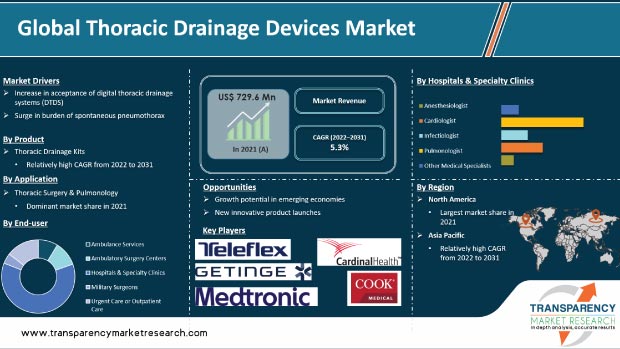
Thoracic or chest drainage helps maintain proper respiratory function and hemodynamic stability by removing air, fluid, or blood from around the heart or lungs. Thoracic drainage systems are primarily utilized in intensive care units (ICUs), emergency departments, and operating rooms. Increase in the acceptance of digital thoracic drainage systems (DTDS), rise in prevalence of CVDs globally, and surge in burden of spontaneous pneumothorax are major factors driving the global thoracic drainage devices market. DTDS is a safe, comfortable, and well-tolerated by patients. Moreover, DTDS helps reduce or eliminate the cost of hospital stay. Novel inventions in thoracic drainage systems are expected to propel the market in the next few years. For instance, in January 2020, Centese, Inc., a developer of advanced drainage systems, announced the completion of the 1st clinical study of its ‘Thoraguard digital drainage system’ used in cardiovascular surgery, at Stanford University Medical Center. Thoraguard is the 1st surgical drainage device to provide automated clog clearance without requiring human intervention, and digitally measures and displays hourly drainage volume and trends to facilitate clinical decision-making after cardiac surgery. Some of the commercially available products in the digital chest drainage devices market are Thopaz (Medela AG), Drentech (REDAX), and Digivent (Millicore AB).
Spontaneous pneumothorax is a one of the major health issues across the globe. Spontaneous pneumothorax is caused due to air in the chest cavity, which can resolve itself; however, most often requires hospital admission and urgent medical care. In a military warfare setting, around 13% to 15% of penetrating wounds related to the thoracic cavity require thoracic intervention including a thoracic catheter. According to an article published in the Canadian Respiratory Journal, in March 2017, primary spontaneous pneumothorax is more common in men than women. This condition occurs in 7.4 to 18 per 100,000 men each year and 1.2 to 6 per 100,000 women. Additionally, incidence of spontaneous pneumothorax is increasing gradually. Various research studies estimate that more than 2 million spontaneous pneumothorax occur globally every year.
Globally, as of August 2022, there have been over 585.0 million confirmed cases of COVID-19, including around 6.4 million deaths, reported to World Health Organization (WHO). However, researches revealed that thoracic drainage systems can significantly help reduce massive spread of COVID-19. Patients suffering from COVID-19 are at high risk of developing acute respiratory distress syndrome (ARDS) needing invasive mechanical ventilation. Furthermore, Barotrauma in COVID-19 patients often leads to pneumothorax, which requires chest tube placement (thoracostomy). Additionally, German Health Alliance (GHA) members, including B. Braun, ATMOS MedizinTechnik GmbH & Co. KG, Getinge, and Dräger, have witnessed a notable increase in the demand for their products, such as thoracic drainage system, from across the world since the beginning of 2020. For instance, ATMOS MedizinTechnik GmbH & Co. KG, an innovative medical device company, announced that production is running at full speed, as product groups such as drainage systems can save lives in the COVID-19 chaos.
In December 2020, Medela LLC, a prominent supplier of breastfeeding accessories and medical vacuum technology, had announced that the filtration system of its ‘Thopaz+ Digital Chest Drainage and Monitoring System’ efficiently blocks passage of aerosolized viral particles, such as SARS-CoV-2. Therefore, respiratory diseases including COVID-19 are expected to open new opportunities in the global thoracic drainage devices market during the forecast period.
In terms of product, the thoracic drainage systems segment held the largest global thoracic drainage devices market share in 2021. The trend is expected to continue during the forecast period owing to increase in demand for thoracic drainage systems in hospitals, urgent care centers, and ambulatory surgery centers. Furthermore, adoption of tube thoracostomy procedures and mobility of thoracic drainage products has increased. It is possible to withdraw drainage sooner in case of digital thoracic drainage system and thereby, discharge patients earlier.
The thoracic drainage kits segment is likely to gain market share during the forecast period owing to higher consumption of chest drainage kit and repetitive use of venous drainage device of the chest wall, or thoracic/chest drainage kit; rising demand for minimally invasive surgeries, high burden of cardiovascular diseases, and increase in burden of spontaneous pneumothorax.
Pleural drainage catheter, also known as thoracic drainage catheter or chest tube or thoracic drainage tube, is a sterile tube that includes a number of drainage holes and is inserted into the pleural space. Demand for thoracic drainage catheter or the chest drainage catheter market in countries in Europe such as the U.S., Germany, and France is increasing due to rise in cardiac and thoracic surgical procedures. In a military warfare setting, around 13% to 15% of penetrating wounds are related to the thoracic cavity, which accounts for around 30% of combat deaths requiring thoracic intervention, including a thoracic drainage catheter.
Thoracic drainage catheter is primarily used for closed chest tube thoracostomies. Cardinal Health, Inc. provides ARGYLE Trocar Catheters (a thoracic trocar catheter) are sterile, single use, disposable devices that consist of a surgically sharp trocar and a clear polyvinyl chloride (PVC) thoracic catheter.
Based on application, the thoracic surgery & pulmonology segment accounted for significant share of the global thoracic drainage devices market in 2021, as thoracic drainage devices are widely used in minimally invasive thoracic surgical procedures for the treatment of pneumothorax, pleural effusions, and empyema disorders. An article in the Journal of Thoracic Disease stated that thoracic surgery is one of the most rapidly developing specialties in all of surgery – both technically and technologically. Currently, around 530,000 general thoracic surgeries are performed yearly in the U.S. by around 4,000 cardiothoracic surgeons.
In terms of end-user, the ambulatory surgery centers segment is expected to register the highest CAGR of 6.7% during the forecast period. ASCs have the ability to improve quality and customer service while simultaneously reducing costs. The ASC vertical is ahead of the curve in identifying favorable avenues for improving healthcare delivery.
The hospitals & specialty clinics segment held the largest share of the global thoracic drainage devices market in 2021. This is ascribed to a surge in the number of hospitals & specialty clinics globally, comparatively higher performance of cardiothoracic surgeries and adoption of thoracic drainage devices in these settings. According to the American Hospital Association (AHA), the U.S. had about 5,564 hospitals in 2017, which increased to 6,093 currently.
The global thoracic drainage devices market forecast during the forecast period reveals that North America is likely to dominate the market due to increase in R&D expenditure, high adoption of novel, improved devices; early availability of advanced technologies; significant spending on healthcare; and the presence of sophisticated healthcare infrastructure and key market participants in the U.S. and Canada. Becton, Dickinson and Company, Teleflex Incorporated, Smiths Medical, Cook Medical, and Cardinal Health, Inc. have a strong presence in the U.S., which is contributing to market growth in North America.
Asia Pacific is likely to gain market share during the forecast period due to larger cardiovascular disease patient population, increase in the geriatric population, developing healthcare infrastructure, and surge in medical tourism. Moreover, increase in interest of key international companies in expanding their production bases and product portfolio across countries in Asia and Southeast Asia, such as South Korea, Malaysia, India, and China due to high population density, is expected to propel the market in the region during the forecast period.
The market report concludes with the company profiles section that includes key information about the major players in the global thoracic drainage devices market. Market participants focus on strategies such as new product launches, mergers & acquisitions (M&As), and public and private partnerships to strengthen their market position. Medtronic plc, Becton, Dickinson and Company (BD), Getinge AB, Teleflex Incorporated, Sterimed Group, ICU medical (Smiths Medical), Cook Medical, Cardinal Health, Inc., Vygon SA, Utah Medical Products, Inc., ATMOS MedizinTechnik GmbH & Co. KG, and Sinapi Biomedical are the prominent players operating in the global market.
Each of these players has been profiled in the thoracic drainage devices market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Market Size Value in 2021 |
US$ 729.6 Mn |
|
Market Forecast Value in 2031 |
More than US$ 1.23 Bn |
|
Compound Annual Growth Rate (CAGR) |
5.3% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2021 |
|
Quantitative Units |
US$ Mn/Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global thoracic drainage devices market was valued at US$ 729.6 Mn in 2021.
The global thoracic drainage devices market is projected to reach more than US$ 1.23 Bn by 2031.
The global thoracic drainage devices market is anticipated to grow at a CAGR of 5.3% from 2022 to 2031.
Increase in acceptance of digital thoracic drainage systems (DTDS), rise in prevalence of CVDs globally, and surge in burden of spontaneous pneumothorax.
The thoracic drainage systems segment accounted for more than 59.0% share of the global thoracic drainage devices market in 2021.
North America is expected to account for major share of the global thoracic drainage devices market during the forecast period.
Medtronic plc, Becton, Dickinson and Company (BD), Getinge AB, Teleflex Incorporated, Sterimed Group, ICU medical (Smiths Medical), Cook Medical, Cardinal Health, Inc., Vygon SA, Utah Medical Products, Inc., ATMOS MedizinTechnik GmbH & Co. KG, and Sinapi Biomedical.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Thoracic Drainage Devices Market
4. Market Overview
4.1. Product Overview
4.2. Global Thoracic Drainage Devices Market Size (US$ Mn) Forecast, 2017-20231
4.3. Global Thoracic Drainage Devices Market Outlook
4.4. Porter's Five Forces Analysis
4.5. Global Thoracic Drainage Devices Market - Product Pricing Analysis
4.6. Global Thoracic Drainage Devices Market - Pricing Analysis for Thoracic Drainage Procedures, by Application
4.7. Global Thoracic Drainage Devices Market - Number of Procedures, by Application, 2017–2031
4.8. Key Mergers & Acquisitions in Medical Device Industry
4.9. PESTLE Analysis
5. Market Dynamics
5.1. Drivers and Restraints Snapshot Analysis
5.2. Drivers
5.3. Restraints
5.4. Opportunities
6. Global Thoracic Drainage Devices Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Product, 2017–2031
6.3.1. Pleural Drainage Catheters
6.3.2. Secured Needles
6.3.3. Unsecured Needles
6.3.4. Thoracic Drainage Kits
6.3.5. Thoracic Drainage Systems
6.3.6. Trocar Drains
6.4. Market Attractiveness Analysis, by Product
7. Global Thoracic Drainage Devices Market Analysis and Forecast, by Application
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Application, 2017–2031
7.3.1. Cardiac Surgery
7.3.2. General Intensive Care & Emergency Medicine
7.3.3. Infectious Diseases
7.3.4. Military/Damage Control/Disaster Medicine
7.3.5. Oncology & Pain Management
7.3.6. Thoracic Surgery & Pulmonology
7.4. Market Attractiveness Analysis, by Application
8. Global Thoracic Drainage Devices Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by End-user, 2017–2031
8.3.1. Ambulance Services
8.3.2. Ambulatory Surgery Centers
8.3.3. Hospitals & Specialty Clinics
8.3.3.1. Anesthesiologist
8.3.3.2. Cardiologist
8.3.3.3. Infectiologist
8.3.3.4. Pulmonologist
8.3.3.5. Other Medical Specialists
8.3.4. Military Surgeons
8.3.5. Urgent Care or Outpatient Care
8.4. Market Attractiveness Analysis, by End-user
9. Global Thoracic Drainage Devices Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Thoracic Drainage Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Product, 2017–2031
10.2.1. Pleural Drainage Catheters
10.2.2. Secured Needles
10.2.3. Unsecured Needles
10.2.4. Thoracic Drainage Kits
10.2.5. Thoracic Drainage Systems
10.2.6. Trocar Drains
10.3. Market Value Forecast, by Application, 2017–2031
10.3.1. Cardiac Surgery
10.3.2. General Intensive Care & Emergency Medicine
10.3.3. Infectious Diseases
10.3.4. Military/Damage Control/Disaster Medicine
10.3.5. Oncology & Pain Management
10.3.6. Thoracic Surgery & Pulmonology
10.4. Market Value Forecast, by End-user, 2017–2031
10.4.1. Ambulance Services
10.4.2. Ambulatory Surgery Centers
10.4.3. Hospitals & Specialty Clinics
10.4.3.1. Anesthesiologist
10.4.3.2. Cardiologist
10.4.3.3. Infectiologist
10.4.3.4. Pulmonologist
10.4.3.5. Other medical specialists
10.4.4. Military Surgeons
10.4.5. Urgent Care or Outpatient Care
10.5. Market Value Forecast, by Country, 2017–2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Product
10.6.2. By Application
10.6.3. By End-user
10.6.4. By Country
11. Europe Thoracic Drainage Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Product, 2017–2031
11.2.1. Pleural Drainage Catheters
11.2.2. Secured Needles
11.2.3. Unsecured Needles
11.2.4. Thoracic Drainage Kits
11.2.5. Thoracic Drainage Systems
11.2.6. Trocar Drains
11.3. Market Value Forecast, by Application, 2017–2031
11.3.1. Cardiac Surgery
11.3.2. General Intensive Care & Emergency Medicine
11.3.3. Infectious Diseases
11.3.4. Military/Damage Control/Disaster Medicine
11.3.5. Oncology & Pain Management
11.3.6. Thoracic Surgery & Pulmonology
11.4. Market Value Forecast, by End-user, 2017–2031
11.4.1. Ambulance Services
11.4.2. Ambulatory Surgery Centers
11.4.3. Hospitals & Specialty Clinics
11.4.3.1. Anesthesiologist
11.4.3.2. Cardiologist
11.4.3.3. Infectiologist
11.4.3.4. Pulmonologist
11.4.3.5. Other medical specialists
11.4.4. Military Surgeons
11.4.5. Urgent Care or Outpatient Care
11.5. Market Value Forecast, by Country/Sub-region, 2017–2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Italy
11.5.5. Spain
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Product
11.6.2. By Application
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Thoracic Drainage Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Product, 2017–2031
12.2.1. Pleural Drainage Catheters
12.2.2. Secured Needles
12.2.3. Unsecured Needles
12.2.4. Thoracic Drainage Kits
12.2.5. Thoracic Drainage Systems
12.2.6. Trocar Drains
12.3. Market Value Forecast, by Application, 2017–2031
12.3.1. Cardiac Surgery
12.3.2. General Intensive Care & Emergency Medicine
12.3.3. Infectious Diseases
12.3.4. Military/Damage Control/Disaster Medicine
12.3.5. Oncology & Pain Management
12.3.6. Thoracic Surgery & Pulmonology
12.4. Market Value Forecast, by End-user, 2017–2031
12.4.1. Ambulance Services
12.4.2. Ambulatory Surgery Centers
12.4.3. Hospitals & Specialty Clinics
12.4.3.1. Anesthesiologist
12.4.3.2. Cardiologist
12.4.3.3. Infectiologist
12.4.3.4. Pulmonologist
12.4.3.5. Other medical specialists
12.4.4. Military Surgeons
12.4.5. Urgent Care or Outpatient Care
12.5. Market Value Forecast, by Country/Sub-region, 2017–2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Product
12.6.2. By Application
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America Thoracic Drainage Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Product, 2017–2031
13.2.1. Pleural Drainage Catheters
13.2.2. Secured Needles
13.2.3. Unsecured Needles
13.2.4. Thoracic Drainage Kits
13.2.5. Thoracic Drainage Systems
13.2.6. Trocar Drains
13.3. Market Value Forecast, by Application, 2017–2031
13.3.1. Cardiac Surgery
13.3.2. General Intensive Care & Emergency Medicine
13.3.3. Infectious Diseases
13.3.4. Military/Damage Control/Disaster Medicine
13.3.5. Oncology & Pain Management
13.3.6. Thoracic Surgery & Pulmonology
13.4. Market Value Forecast, by End-user, 2017–2031
13.4.1. Ambulance Services
13.4.2. Ambulatory Surgery Centers
13.4.3. Hospitals & Specialty Clinics
13.4.3.1. Anesthesiologist
13.4.3.2. Cardiologist
13.4.3.3. Infectiologist
13.4.3.4. Pulmonologist
13.4.3.5. Other medical specialists
13.4.4. Military Surgeons
13.4.5. Urgent Care or Outpatient Care
13.5. Market Value Forecast, by Country/Sub-region, 2017–2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Product
13.6.2. By Application
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Thoracic Drainage Devices Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Product, 2017–2031
14.2.1. Pleural Drainage Catheters
14.2.2. Secured Needles
14.2.3. Unsecured Needles
14.2.4. Thoracic Drainage Kits
14.2.5. Thoracic Drainage Systems
14.2.6. Trocar Drains
14.3. Market Value Forecast, by Application, 2017–2031
14.3.1. Cardiac Surgery
14.3.2. General Intensive Care & Emergency Medicine
14.3.3. Infectious Diseases
14.3.4. Military/Damage Control/Disaster Medicine
14.3.5. Oncology & Pain Management
14.3.6. Thoracic Surgery & Pulmonology
14.4. Market Value Forecast, by End-user, 2017–2031
14.4.1. Ambulance Services
14.4.2. Ambulatory Surgery Centers
14.4.3. Hospitals & Specialty Clinics
14.4.3.1. Anesthesiologist
14.4.3.2. Cardiologist
14.4.3.3. Infectiologist
14.4.3.4. Pulmonologist
14.4.3.5. Other medical specialists
14.4.4. Military Surgeons
14.4.5. Urgent Care or Outpatient Care
14.5. Market Value Forecast, by Country/Sub-region, 2017–2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Product
14.6.2. By Application
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player – Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2021
15.3. Company Profiles
15.3.1. Teleflex Incorporated
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Financial Overview
15.3.1.4. SWOT Analysis
15.3.2. Medtronic
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. Strategic Overview
15.3.2.5. SWOT Analysis
15.3.3. Cook Medical
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Strategic Overview
15.3.3.4. SWOT Analysis
15.3.4. ICU medical (Smiths Medical)
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. Strategic Overview
15.3.4.5. SWOT Analysis
15.3.5. Cardinal Health, Inc.
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. Strategic Overview
15.3.5.5. SWOT Analysis
15.3.6. Becton Dickinson and Company
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Financial Overview
15.3.6.4. SWOT Analysis
15.3.7. Getinge AB
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. Strategic Overview
15.3.7.5. SWOT Analysis
15.3.8. Stermid Group
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. SWOT Analysis
15.3.9. Vygon SA
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. SWOT Analysis
15.3.10. Utah Medical Products, Inc.
15.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.10.2. Product Portfolio
15.3.10.3. Financial Overview
15.3.10.4. SWOT Analysis
15.3.11. ATMOS MedizinTechnik GmbH & Co. KG
15.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.11.2. Product Portfolio
15.3.11.3. SWOT Analysis
15.3.12. Sinapi Biomedical
15.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.12.2. Product Portfolio
15.3.12.3. SWOT Analysis
List of Tables
Table 01: Global Thoracic Drainage Devices Market - Number of Procedures Forecast, by Application, 2017–2031
Table 02: Global Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 03: Global Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 04: Global Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 05: Global Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Hospitals & Specialty Clinics, 2017–2031
Table 06: Global Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 07: North America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 08: North America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 09: North America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 10: North America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Hospitals & Specialty Clinics, 2017–2031
Table 11: North America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 12: Europe Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 13: Europe Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 14: Europe Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 15: Europe Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Hospitals & Specialty Clinics, 2017–2031
Table 16: Europe Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 17: Asia Pacific Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 18: Asia Pacific Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 19: Asia Pacific Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 20: Asia Pacific Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Hospitals & Specialty Clinics, 2017–2031
Table 21: Asia Pacific Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 22: Latin America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 23: Latin America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 24: Latin America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 25: Latin America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Hospitals & Specialty Clinics, 2017–2031
Table 26: Latin America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 27: Middle East & Africa Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 28: Middle East & Africa Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 29: Middle East & Africa Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 30: Middle East & Africa Thoracic Drainage Devices Market Value (US$ Mn) Forecast, by Hospitals & Specialty Clinics, 2017–2031
List of Figures
Figure 01: Global Thoracic Drainage Devices (CKD) Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Global Thoracic Drainage Devices Market Value Share, by Product (2021)
Figure 03: Global Thoracic Drainage Devices Market Value Share, by Application (2021)
Figure 04: Global Thoracic Drainage Devices Market Value Share, by End-user (2021)
Figure 05: Global Thoracic Drainage Devices Market Value Share, by Country/Region (2021)
Figure 06: Global Thoracic Drainage Devices Market Analysis and Forecast, by Product, 2021 and 2031
Figure 07: Global Thoracic Drainage Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 08: Global Thoracic Drainage Devices Market (US$ Mn), by Pleural Drainage Catheters, 2017–2031
Figure 09: Global Thoracic Drainage Devices Market (US$ Mn), by Secured Needles, 2017-2031
Figure 10: Global Thoracic Drainage Devices Market (US$ Mn), by Unsecured Needles, 2017-2031
Figure 11: Global Thoracic Drainage Devices Market (US$ Mn), by Thoracic Drainage Kits, 2017-2031
Figure 12: Global Thoracic Drainage Devices Market (US$ Mn), by Thoracic Drainage Systems, 2017-2031
Figure 13: Global Thoracic Drainage Devices Market (US$ Mn), by Trocar Drains, 2017-2031
Figure 14: Global Thoracic Drainage Devices Market Analysis and Forecast, by Application, 2021 and 2031
Figure 15: Global Thoracic Drainage Devices Market Attractiveness Analysis, by Application, 2022–2031
Figure 16: Global Thoracic Drainage Devices Market (US$ Mn), by Cardiac Surgery, 2017–2031
Figure 17: Global Thoracic Drainage Devices Market (US$ Mn), by General Intensive Care & Emergency Medicine, 2017-2031
Figure 18: Global Thoracic Drainage Devices Market (US$ Mn), by Infectious Diseases, 2017-2031
Figure 19: Global Thoracic Drainage Devices Market (US$ Mn), by Military/Damage Control/Disaster Medicine, 2017-2031
Figure 20: Global Thoracic Drainage Devices Market (US$ Mn), by Oncology & Pain Management, 2017-2031
Figure 21: Global Thoracic Drainage Devices Market (US$ Mn), by Thoracic Surgery & Pulmonology, 2017-2031
Figure 22: Global Thoracic Drainage Devices Market Analysis and Forecast, by End-user, 2021 and 2031
Figure 23: Global Thoracic Drainage Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 24: Global Thoracic Drainage Devices Market (US$ Mn), by Ambulance Services, 2017–2031
Figure 25: Global Thoracic Drainage Devices Market (US$ Mn), by Ambulatory Surgery Centers, 2017-2031
Figure 26: Global Thoracic Drainage Devices Market (US$ Mn), by Hospitals & Specialty Clinics, 2017-2031
Figure 27: Global Thoracic Drainage Devices Market (US$ Mn), by Military Surgeons, 2017-2031
Figure 28: Global Thoracic Drainage Devices Market (US$ Mn), by Urgent Care or Outpatient Care, 2017-2031
Figure 29: Global Thoracic Drainage Devices Market Analysis and Forecast, by Region, 2021 and 2031
Figure 30: Global Thoracic Drainage Devices Market Attractiveness Analysis, by Region, 2022–2031
Figure 31: North America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 32: North America Thoracic Drainage Devices Market Analysis and Forecast, by Product, 2021 and 2031
Figure 33: North America Thoracic Drainage Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 34: North America Thoracic Drainage Devices Market Analysis and Forecast, by Application, 2021 and 2031
Figure 35: North America Thoracic Drainage Devices Market Attractiveness Analysis, by Application, 2022–2031
Figure 36: North America Thoracic Drainage Devices Market Analysis and Forecast, by End-user, 2021 and 2031
Figure 37: North America Thoracic Drainage Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 38: North America Thoracic Drainage Devices Market Analysis and Forecast, by Country, 2021 and 2031
Figure 39: North America Thoracic Drainage Devices Market Attractiveness Analysis, by Country, 2022–2031
Figure 40: Europe Thoracic Drainage Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 41: Europe Thoracic Drainage Devices Market Analysis and Forecast, by Product, 2021 and 2031
Figure 42: Europe Thoracic Drainage Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 43: Europe Thoracic Drainage Devices Market Analysis and Forecast, by Application. 2021 and 2031
Figure 44: Europe Thoracic Drainage Devices Market Attractiveness Analysis, by Application, 2022–2031
Figure 45: Europe Thoracic Drainage Devices Market Analysis and Forecast, by End-user, 2021 and 2031
Figure 46: Europe Thoracic Drainage Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 47: Europe Thoracic Drainage Devices Market Analysis and Forecast, by Country/Sub-region, 2021 and 2031
Figure 48: Europe Thoracic Drainage Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 49: Asia Pacific Thoracic Drainage Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 50: Asia Pacific Thoracic Drainage Devices Market Analysis and Forecast, by Product, 2021 and 2031
Figure 51: Asia Pacific Thoracic Drainage Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 52: Asia Pacific Thoracic Drainage Devices Market Analysis and Forecast, by Application 2021 and 2031
Figure 53: Asia Pacific Thoracic Drainage Devices Market Attractiveness Analysis, by Application, 2022–2031
Figure 54: Asia Pacific Thoracic Drainage Devices Market Analysis and Forecast, by End-user, 2021 and 2031
Figure 55: Asia Pacific Thoracic Drainage Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 56: Asia Pacific Thoracic Drainage Devices Market Analysis and Forecast, by Country/Sub-region, 2021 and 2031
Figure 57: Asia Pacific Thoracic Drainage Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 58: Latin America Thoracic Drainage Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 59: Latin America Thoracic Drainage Devices Market Analysis and Forecast, by Product, 2021 and 2031
Figure 60: Latin America Thoracic Drainage Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 61: Latin America Thoracic Drainage Devices Market Analysis and Forecast, by Application 2021 and 2031
Figure 62: Latin America Thoracic Drainage Devices Market Attractiveness Analysis, by Application, 2022–2031
Figure 63: Latin America Thoracic Drainage Devices Market Analysis and Forecast, by Distribution Channel, 2021 and 2031
Figure 64: Latin America Thoracic Drainage Devices Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 65: Latin America Thoracic Drainage Devices Market Analysis and Forecast, by Country/Sub-region, 2021 and 2031
Figure 66: Latin America Thoracic Drainage Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 67: Middle East & Africa Thoracic Drainage Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 68: Middle East & Africa Thoracic Drainage Devices Market Analysis and Forecast, by Product, 2021 and 2031
Figure 69: Middle East & Africa Thoracic Drainage Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 70: Middle East & Africa Thoracic Drainage Devices Market Analysis and Forecast, by Application 2021 and 2031
Figure 71: Middle East & Africa Thoracic Drainage Devices Market Attractiveness Analysis, by Application, 2022–2031
Figure 72: Middle East & Africa Thoracic Drainage Devices Market Analysis and Forecast, by End-user, 2021 and 2031
Figure 73: Middle East & Africa Thoracic Drainage Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 74: Global Thoracic Drainage Device Market Share Analysis/Ranking, by Company, 2021