Reports
Reports
Analysts’ Viewpoint
The global radiopharmaceutical theranostics market is expected to be driven by rise in prevalence of cancer & other diseases and advancements in radiopharmaceutical technology during the forecast period. Surge in demand for personalized medicine is another factor fueling market development. Increase in usage of PET-CT and SPECT-CT imaging technologies, which offer high-resolution and accurate imaging of specific tissues and organs, is likely to accelerate market expansion in the next few years.
Development of novel radiopharmaceuticals that can target specific biomarkers associated with certain diseases offers significant opportunities to market players. Leading players are focusing on research & development of novel theranostic agents in order to increase market share.
Radiopharmaceutical theranostics is an approach to medical treatment that involves the use of radiopharmaceuticals for both diagnosis and therapy of various diseases, including cancer. Radiopharmaceuticals are compounds that contain a radioactive component that can be used to target and kill cancer cells.
The term "theranostics" is a combination of the words "therapy" and "diagnostics," and refers to the usage of the same radiopharmaceuticals for both diagnostic imaging and therapeutic treatment. This approach offers several advantages, including increased accuracy in diagnosis, targeted therapy that can improve outcomes while minimizing side effects, and the potential for more personalized treatment options.
One of the key benefits of radiopharmaceutical theranostics is that it allows for the targeted delivery of therapeutic agents to cancer cells, while sparing healthy tissue. This can result in fewer side effects compared to traditional chemotherapy and radiation therapy, which can damage healthy tissue along with cancerous cells.
Cancer is a complex disease caused by uncontrolled growth and division of abnormal cells in the body. It could occur in almost any part of the body and spread to other parts through the bloodstream or lymphatic system. The incidence of cancer is rising across the world. According to the World Health Organization (WHO), cancer is the second leading cause of death globally, accounting for an estimated 9.6 million deaths in 2018.
According to the American Cancer Society, an estimated 1.9 million new cancer cases and 608,570 cancer deaths were recorded in the U.S. in 2021 alone. Additionally, the National Cancer Institute (NCI) indicated that the number of people living with cancer in the U.S. is expected to reach 22.2 million by 2030, a significant increase from the 16.9 million cancer survivors in 2019.
Rise in prevalence of cancer has driven the development of new and innovative cancer treatments, including radiopharmaceutical theranostics. Radiopharmaceuticals are compounds that contain a radioactive isotope, which emits radiation as it decays. When administered into the body, these compounds can be used to identify the presence and location of cancer cells through a process called molecular imaging. Once the cancer is identified, the same radiopharmaceutical can be used to deliver targeted radiation therapy directly to the cancer cells, killing them without damaging surrounding healthy tissue.
Radiopharmaceutical theranostics is especially useful for treating cancer that has spread to multiple locations in the body, as the targeted radiation could reach cancer cells wherever they are located. It is also effective for treating cancer that is resistant to traditional chemotherapy or radiation therapy.
Development of radiopharmaceutical theranostics is driven, in part, by rise in prevalence of cancer as well as by advances in imaging technology and molecular biology. As the understanding of cancer biology and the mechanisms of cancer progression has improved, it has become possible to develop more targeted and effective cancer treatments, including radiopharmaceutical theranostics.
The field of radiopharmaceutical theranostics has evolved rapidly in the past few years, with new developments and innovations driving growth and investment in the market. Surge in investment in the development of novel theranostic agents is a major factor driving the global radiopharmaceutical theranostics market size.
Radiopharmaceutical theranostics is a unique approach to cancer treatment that uses targeted radiation to both diagnose and treat cancer. It involves the usage of radiopharmaceuticals. These compounds can be used for molecular imaging to identify the presence and location of cancer cells, as well as for targeted radiation therapy to deliver precise treatment directly to cancer cells.
The development of new theranostic agents is critical to the advancement of radiopharmaceutical theranostics. As our understanding of cancer biology and the mechanisms of cancer progression continues to improve, it is essential to develop new compounds that can effectively target cancer cells and minimize damage to healthy tissue.
Investment in research & development for new theranostic agents has increased in the past few years, driven by the growing demand for personalized and targeted cancer treatments. These investments are coming from various sources, including government agencies, private investors, and pharmaceutical companies.
Government agencies, such as the NIH and the National Science Foundation (NSF), provide funding for research projects that aim to develop new theranostic agents. Additionally, the U.S. Government recently passed legislation aimed at increasing funding for cancer research and drug development, which is expected to drive investment in this market.
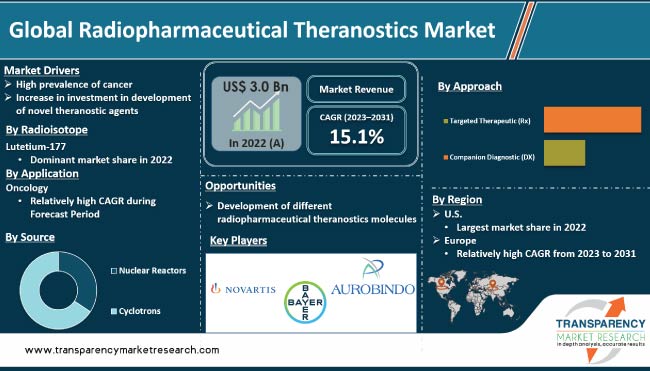
In terms of radioisotope, the lutetium 177 segment dominated the global radiopharmaceutical theranostics industry in 2022. Lutetium-177 (Lu-177) is a radioactive isotope of the element lutetium. It is a beta-emitting radionuclide; it emits beta particles during radioactive decay. Lu-177 has a half-life of 6.7 days, which is long enough for it to be used in medical applications, but short enough to limit patient exposure to radiation.
Lu-177 has been investigated for its potential in targeted radionuclide therapy (TRT) and imaging in the field of nuclear medicine. It can be coupled with a targeting molecule to create a radiopharmaceutical agent that specifically binds to certain types of cancer cells, delivering therapeutic doses of radiation directly to the cancerous cells while sparing normal tissues.
Lutetium-177 is a therapeutic radiopharmaceutical that has shown promising results in the treatment of various types of cancer, including neuroendocrine tumors and prostate cancer. High efficacy, relatively low toxicity, and convenient production are driving market demand. The lutetium-177 segment is expected to dominate the radiopharmaceutical theranostics market in the next few years due to surge in demand and increase in adoption.
Based on application, the oncology segment accounted for the largest global radiopharmaceutical theranostics market share in 2022. Radiopharmaceuticals are increasingly being used in the field of oncology for targeted imaging and therapy of cancer cells. By using radiopharmaceuticals, physicians can more accurately locate and diagnose tumors, as well as deliver targeted radiation therapy to the cancerous cells, while minimizing damage to healthy tissues. Development of more effective radiopharmaceuticals and increase in adoption in targeted therapy in the treatment of cancer is expected to augment the oncology segment.
In terms of source, the cyclotrons segment dominated the global radiopharmaceutical theranostics market in 2022. Cyclotrons are typically used to produce radioisotopes such as F-18, C-11, and N-13, which are used in PET imaging. These radioisotopes have relatively short half-live and need to be produced on-site at the medical facility, where they will be used. Hence, cyclotrons are often located at hospitals and imaging centers.
Usage of cyclotron-produced radiopharmaceuticals has increased rapidly in the past few years, as PET imaging has become increasingly important for the diagnosis and staging of many different types of cancer. Rise in demand for PET imaging agents is expected to propel the cyclotrons segment.
Based on approach, the targeted therapeutic (Rx) segment led the global radiopharmaceutical theranostics market in 2022. Targeted therapeutic (Rx) approaches have shown promising results in the treatment of various types of cancer, including neuroendocrine tumors, prostate cancer, and some types of lymphoma. One of the most commonly used radioisotopes for targeted therapy is Lutetium-177 (Lu-177), which has shown efficacy in clinical trials for the treatment of neuroendocrine tumors and prostate cancer.
Growth of the targeted therapeutic (Rx) segment can be attributed to increase in demand for personalized cancer treatment and development of new targeting molecules that allow for more precise and effective delivery of radiation therapy.
In terms of radiotracer type, the peptidic segment led the global radiopharmaceutical theranostics market in 2022. Peptidic radiopharmaceuticals have shown promising results in the diagnosis and treatment of various types of cancer, particularly neuroendocrine tumors. The most commonly used peptidic radiopharmaceuticals are DOTATOC, DOTATATE, and DOTANOC, which target the somatostatin receptor, and PSMA-617, which targets the prostate-specific membrane antigen.
Growth of the peptidic segment can be attributed to increase in research & development in peptide therapeutics and development of new imaging and treatment options for neuroendocrine tumors and prostate cancer.
Based on end-user, the hospitals segment led the global radiopharmaceutical theranostics market in 2022. Hospitals are one of the primary end-users of radiopharmaceuticals, as these are the preferred settings for diagnostic and therapeutic procedures involving radiotracers.
Hospitals use radiopharmaceuticals for a range of applications, including radiopharmaceutical diagnosis and staging of cancer, as well as treatment of various types of cancer and other diseases. Usage of radiopharmaceuticals in hospitals has increased rapidly in the past few years, as new imaging and therapeutic options have become available and the demand for personalized medicine has increased.
Growth of the hospitals segment can also be ascribed to factors such as increase in healthcare spending, rise in incidence of cancer, and surge in adoption of nuclear medicine and molecular imaging techniques in clinical practice.
As per radiopharmaceutical theranostics market trends, the U.S. accounted for the largest share of the global radiopharmaceutical theranostics market in 2022. The country has been at the forefront of research & development in the field of radiopharmaceutical theranostics, and has a highly developed healthcare system that is equipped to support the use of radiotracers in clinical practice. Additionally, the U.S. has a large and aging population, which has contributed to rise in demand for diagnostic and therapeutic options for cancer and other diseases.
Increase in healthcare spending, favorable regulatory policies, and presence of key industry players are the other factors contributing to the growth of the radiopharmaceutical theranostics market in the U.S.
Growth of the radiopharmaceutical theranostics market in the EU is ascribed to usage of radioactive isotopes to diagnose and treat diseases. Radiopharmaceuticals are used in nuclear medicine to produce images of the body's organs and tissues, and to deliver targeted radiation therapy to cancer cells. The market is driven by factors such as increase in incidence of cancer, rise in adoption of nuclear medicine, and advancements in radiopharmaceuticals. Additionally, surge in demand for personalized medicine and increase in usage of radiopharmaceuticals in clinical trials contribute to the growth of the market in EU.
This report provides profiles of leading players operating in the global radiopharmaceutical theranostics market. These include Advanced Accelerator Applications (Novartis AG), Aurobindo Pharma, Bayer AG, Blue Earth Diagnostics (Bracco), Cardinal Health, Clarity Pharmaceuticals, GE Healthcare, Jubliant Radiopharma, Navidea Biopharmaceuticals, Inc., SOFIE, Telix Pharmeceuticals, and Lantheus Medical Imaging. These players engage in merger & acquisition, strategic collaborations, and new product launches to expand presence and increase market share.
The report profiles the top players based on various factors including a company overview, financial summary, strategies, product portfolio, segments, and recent advancements in the market.
|
Attribute |
Detail |
|
Size in 2022 |
US$ 3.0 Bn |
|
Forecast (Value) in 2031 |
More than US$ 12.4 Bn |
|
Growth Rate (CAGR) |
15.1% |
|
Forecast Period |
2023–2031 |
|
Historical Data Available for |
2017–2022 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global industry was valued at US$ 3.0 Bn in 2022.
It is projected to reach more than US$ 12.4 Bn by 2031.
The CAGR is anticipated to be 15.1% from 2023 to 2031.
The lutetium-177 segment held the largest share in 2022.
The U.S. is expected to account for significant share during the forecast period.
Advanced Accelerator Applications (Novartis AG), Aurobindo Pharma, Bayer AG, Blue Earth Diagnostics (Bracco), Cardinal Health, Clarity Pharmaceuticals, GE Healthcare, Jubliant Radiopharma, Navidea Biopharmaceuticals, Inc., SOFIE, Telix Pharmeceuticals, and Lantheus Medical Imaging are the prominent players in the market.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Radiopharmaceutical Theranostics Market
4. Market Overview
4.1. Introduction
4.1.1. Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, 2017-2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Regulatory Scenario
5.2. Key Distribution Strategies
5.3. Pricing Analysis
5.4. Patents on Radiotracers
5.5. Technological Advancements in Radiopharmaceutical Theranostics
5.6. Radiotracer Type - Overview
5.7. COVID-19 Pandemic Impact on Industry
6. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by Radioisotope
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Radioisotope, 2017-2031
6.3.1. Technetium-99
6.3.2. Gallium-68
6.3.3. Iodine-131
6.3.4. Radium-223
6.3.5. Fluorine-18
6.3.6. Yttrium-90
6.3.7. Lutetium-177
6.3.8. Copper-67 & 64
6.3.9. Samarium-153
6.3.10. Others
6.4. Market Attractiveness Analysis, by Radioisotope
7. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by Application
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Application, 2017-2031
7.3.1. Oncology
7.3.2. Cardiology
7.3.3. Others
7.4. Market Attractiveness Analysis, by Application
8. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by Source
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by Source, 2017-2031
8.3.1. Nuclear Reactors
8.3.2. Cyclotrons
8.4. Market Attractiveness Analysis, by Source
9. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by Approach
9.1. Introduction & Definition
9.2. Key Findings/Developments
9.3. Market Value Forecast, by Approach, 2017-2031
9.3.1. Targeted Therapeutic (Rx)
9.3.2. Companion Diagnostic (DX)
9.4. Market Attractiveness Analysis, by Approach
10. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by Radiotracer Type
10.1. Introduction & Definition
10.2. Key Findings/Developments
10.3. Market Value Forecast, by Radiotracer Type, 2017-2031
10.3.1. Peptidic
10.3.2. Non-peptidic
10.4. Market Attractiveness Analysis, by Radiotracer Type
11. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by End-user
11.1. Introduction & Definition
11.2. Key Findings/Developments
11.3. Market Value Forecast, by End-user, 2017-2031
11.3.1. Hospitals
11.3.2. Academic & Research Institutes
11.3.3. Others
11.4. Market Attractiveness Analysis, by End-user
12. Global Radiopharmaceutical Theranostics Market Analysis and Forecast, by Region
12.1. Key Findings
12.2. Market Value Forecast, by Region, 2017-2031
12.2.1. U.S.
12.2.2. Europe
12.2.3. Rest of the World
12.3. Market Attractiveness Analysis, by Region
13. U.S. Radiopharmaceutical Theranostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Radioisotope, 2017-2031
13.2.1. Technetium-99
13.2.2. Gallium-68
13.2.3. Iodine-131
13.2.4. Radium-223
13.2.5. Fluorine-18
13.2.6. Yttrium-90
13.2.7. Lutetium-177
13.2.8. Copper-67 & 64
13.2.9. Samarium-153
13.2.10. Others
13.3. Market Value Forecast, by Application, 2017-2031
13.3.1. Oncology
13.3.2. Cardiology
13.3.3. Others
13.4. Market Value Forecast, by Source, 2017-2031
13.4.1. Nuclear Reactors
13.4.2. Cyclotrons
13.5. Market Value Forecast, by Approach, 2017-2031
13.5.1. Targeted Therapeutic (Rx)
13.5.2. Companion Diagnostic (DX)
13.6. Market Value Forecast, by Radiotracer Type, 2017-2031
13.6.1. Peptidic
13.6.2. Non-peptidic
13.7. Market Value Forecast, by End-user, 2017-2031
13.7.1. Hospitals
13.7.2. Academic & Research Institutes
13.7.3. Others
13.8. Market Attractiveness Analysis
13.8.1. By Radioisotope
13.8.2. By Application
13.8.3. By Source
13.8.4. By Approach
13.8.5. By Radiotracer Type
13.8.6. By End-user
14. Europe Radiopharmaceutical Theranostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Radioisotope, 2017-2031
14.2.1. Technetium-99
14.2.2. Gallium-68
14.2.3. Iodine-131
14.2.4. Radium-223
14.2.5. Fluorine-18
14.2.6. Yttrium-90
14.2.7. Lutetium-177
14.2.8. Copper-67 & 64
14.2.9. Samarium-153
14.2.10. Others
14.3. Market Value Forecast, by Application, 2017-2031
14.3.1. Oncology
14.3.2. Cardiology
14.3.3. Others
14.4. Market Value Forecast, by Source, 2017-2031
14.4.1. Nuclear Reactors
14.4.2. Cyclotrons
14.5. Market Value Forecast, by Approach, 2017-2031
14.5.1. Targeted Therapeutic (Rx)
14.5.2. Companion Diagnostic (DX)
14.6. Market Value Forecast, by Radiotracer Type, 2017-2031
14.6.1. Peptidic
14.6.2. Non-peptidic
14.7. Market Value Forecast, by End-user, 2017-2031
14.7.1. Hospitals
14.7.2. Academic & Research Institutes
14.7.3. Others
14.8. Market Value Forecast, by Country/Sub-region, 2017-2031
14.8.1. Germany
14.8.2. U.K.
14.8.3. France
14.8.4. Italy
14.8.5. Spain
14.8.6. Rest of Europe
14.9. Market Attractiveness Analysis
14.9.1. By Radioisotope
14.9.2. By Application
14.9.3. By Source
14.9.4. By Approach
14.9.5. By Radiotracer Type
14.9.6. By End-user
14.9.7. By Country/Sub-region
15. Rest of the World Radiopharmaceutical Theranostics Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Radioisotope, 2017-2031
15.2.1. Technetium-99
15.2.2. Gallium-68
15.2.3. Iodine-131
15.2.4. Radium-223
15.2.5. Fluorine-18
15.2.6. Yttrium-90
15.2.7. Lutetium-177
15.2.8. Copper-67 & 64
15.2.9. Samarium-153
15.2.10. Others
15.3. Market Value Forecast, by Application, 2017-2031
15.3.1. Oncology
15.3.2. Cardiology
15.3.3. Others
15.4. Market Value Forecast, by Source, 2017-2031
15.4.1. Nuclear Reactors
15.4.2. Cyclotrons
15.5. Market Value Forecast, by Approach, 2017-2031
15.5.1. Targeted Therapeutic (Rx)
15.5.2. Companion Diagnostic (DX)
15.6. Market Value Forecast, by Radiotracer Type, 2017-2031
15.6.1. Peptidic
15.6.2. Non-peptidic
15.7. Market Value Forecast, by End-user, 2017-2031
15.7.1. Hospitals
15.7.2. Academic & Research Institutes
15.7.3. Others
15.8. Market Attractiveness Analysis
15.8.1. By Radioisotope
15.8.2. By Application
15.8.3. By Source
15.8.4. By Approach
15.8.5. By Radiotracer Type
15.8.6. By End-user
16. Competition Landscape
16.1. Market Player Competition Matrix (by tier and size of companies)
16.2. Market Share Analysis, by Company (2022)
16.3. Company Profiles
16.3.1. Advanced Accelerator Applications (Novartis AG)
16.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.1.2. Product Portfolio
16.3.1.3. Financial Overview
16.3.1.4. SWOT Analysis
16.3.1.5. Strategic Overview
16.3.2. Aurobindo Pharma
16.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.2.2. Product Portfolio
16.3.2.3. Financial Overview
16.3.2.4. SWOT Analysis
16.3.2.5. Strategic Overview
16.3.3. Bayer AG
16.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.3.2. Product Portfolio
16.3.3.3. Financial Overview
16.3.3.4. SWOT Analysis
16.3.3.5. Strategic Overview
16.3.4. Blue Earth Diagnostics (Bracco)
16.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.4.2. Product Portfolio
16.3.4.3. Financial Overview
16.3.4.4. SWOT Analysis
16.3.4.5. Strategic Overview
16.3.5. Cardinal Health
16.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.5.2. Product Portfolio
16.3.5.3. Financial Overview
16.3.5.4. SWOT Analysis
16.3.5.5. Strategic Overview
16.3.6. Clarity Pharmaceuticals
16.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.6.2. Product Portfolio
16.3.6.3. Financial Overview
16.3.6.4. SWOT Analysis
16.3.6.5. Strategic Overview
16.3.7. GE Healthcare
16.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.7.2. Product Portfolio
16.3.7.3. Financial Overview
16.3.7.4. SWOT Analysis
16.3.7.5. Strategic Overview
16.3.8. Jubliant Radiopharma
16.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.8.2. Product Portfolio
16.3.8.3. Financial Overview
16.3.8.4. SWOT Analysis
16.3.8.5. Strategic Overview
16.3.9. Lantheus Medical Imaging
16.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.9.2. Product Portfolio
16.3.9.3. Financial Overview
16.3.9.4. SWOT Analysis
16.3.9.5. Strategic Overview
16.3.10. Navidea Biopharmaceuticals, Inc.
16.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.10.2. Product Portfolio
16.3.10.3. Financial Overview
16.3.10.4. SWOT Analysis
16.3.10.5. Strategic Overview
16.3.11. SOFIE
16.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.11.2. Product Portfolio
16.3.11.3. Financial Overview
16.3.11.4. SWOT Analysis
16.3.11.5. Strategic Overview
16.3.12. Telix Pharmaceuticals
16.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.12.2. Product Portfolio
16.3.12.3. Financial Overview
16.3.12.4. SWOT Analysis
16.3.12.5. Strategic Overview
List of Tables
Table 01: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radioisotope, 2017–2031
Table 02: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 03: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Source, 2017–2031
Table 04: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Approach, 2017–2031
Table 05: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radiotracer Type, 2017–2031
Table 06: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 07: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Country/Region, 2017–2031
Table 08: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radioisotope, 2017–2031
Table 09: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 10: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Source, 2017–2031
Table 11: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Approach, 2017–2031
Table 12: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radiotracer Type, 2017–2031
Table 13: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 14: Europe Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 15: Europe Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radioisotope, 2017–2031
Table 16: Europe Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 17: Europe Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Source, 2017–2031
Table 18: Europe Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Approach, 2017–2031
Table 19: EU Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radiotracer Type, 2017–2031
Table 20: EU Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 21: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radioisotope, 2017–2031
Table 22: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Application, 2017–2031
Table 23: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Source, 2017–2031
Table 24: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Approach, 2017–2031
Table 25: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by Radiotracer Type, 2017–2031
Table 26: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
List of Figures
Figure 01: Global Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Global Radiopharmaceutical Theranostics Market Value Share, by Radioisotope, 2022
Figure 03: Global Radiopharmaceutical Theranostics Market Value Share, by Application, 2022
Figure 04: Global Radiopharmaceutical Theranostics Market Value Share, by Source, 2022
Figure 05: Global Radiopharmaceutical Theranostics Market Value Share, by Approach, 2022
Figure 06: Global Radiopharmaceutical Theranostics Market Value Share, by End-user, 2022
Figure 07: Global Radiopharmaceutical Theranostics Market Value Share, by Radiotracer Type, 2022
Figure 08: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Radioisotope, 2022 and 2031
Figure 09: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radioisotope, 2023–2031
Figure 10: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Technetium-99, 2017–2031
Figure 11: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Gallium-68, 2017–2031
Figure 12: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Iodine-131, 2017–2031
Figure 13: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Radium-223, 2017–2031
Figure 14: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Fluorine-18, 2017–2031
Figure 15: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Yttrium-90, 2017–2031
Figure 16: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Lutetium-177, 2017–2031
Figure 17: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Copper-67 & 64, 2017–2031
Figure 18: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Samarium-153, 2017–2031
Figure 19: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Others, 2017–2031
Figure 20: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Application, 2022 and 2031
Figure 21: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, Application, 2023–2031
Figure 22: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Oncology, 2017–2031
Figure 23: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Cardiology, 2017–2031
Figure 24: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Others, 2017–2031
Figure 25: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Source, 2022 and 2031
Figure 26: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, Source, 2023–2031
Figure 27: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Nuclear Reactors, 2017–2031
Figure 28: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Cyclotrons, 2017–2031
Figure 29: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Approach, 2022 and 2031
Figure 30: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, Approach, 2023–2031
Figure 31: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Targeted Therapeutic (Rx), 2017–2031
Figure 32: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Companion Diagnostic (DX), 2017–2031
Figure 33: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Radiotracer Type, 2022 and 2031
Figure 34: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radiotracer Type, 2023–2031
Figure 35: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Peptidic, 2017–2031
Figure 36: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Non-peptidic, 2017–2031
Figure 37: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by End-user 2022 and 2031
Figure 38: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, End-user, 2023–2031
Figure 39: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Hospitals, 2017–2031
Figure 40: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Academic & Research Institutes, 2017–2031
Figure 41: Global Radiopharmaceutical Theranostics Market Revenue (US$ Mn), by Others, 2017–2031
Figure 42: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Country/Region, 2022 and 2031
Figure 43: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, by Country/Region, 2023–2031
Figure 44: U.S. Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 45: U.S. Radiopharmaceutical Theranostics Market Value Share Analysis, by Radioisotope, 2022 and 2031
Figure 46: U.S. Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radioisotope, 2023–2031
Figure 47: U.S. Radiopharmaceutical Theranostics Market Value Share Analysis, by Application, 2022 and 2031
Figure 48: U.S. Radiopharmaceutical Theranostics Market Attractiveness Analysis, Application, 2023–2031
Figure 49: U.S. Radiopharmaceutical Theranostics Market Value Share Analysis, by Source 2022, and 2031
Figure 50: U.S. Radiopharmaceutical Theranostics Market Attractiveness Analysis, Source, 2023–2031
Figure 51: U.S. Radiopharmaceutical Theranostics Market Value Share Analysis, by Approach, 2022 and 2031
Figure 52: U.S. Radiopharmaceutical Theranostics Market Attractiveness Analysis, Approach, 2023–2031
Figure 53: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by Radiotracer Type, 2022 and 2031
Figure 54: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radiotracer Type, 2023–2031
Figure 55: Global Radiopharmaceutical Theranostics Market Value Share Analysis, by End-user, 2022 and 2031
Figure 56: Global Radiopharmaceutical Theranostics Market Attractiveness Analysis, End-user, 2023–2031
Figure 57: Europe Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 58: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 59: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 60: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by Radioisotope, 2022 and 2031
Figure 61: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radioisotope, 2023–2031
Figure 62: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by Application, 2022 and 2031
Figure 63: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, Application, 2023–2031
Figure 64: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by Source, 2022 and 2031
Figure 65: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, Source, 2023–2031
Figure 66: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by Approach, 2022 and 2031
Figure 67: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, Approach, 2023–2031
Figure 68: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by Radiotracer Type, 2022 and 2031
Figure 69: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radiotracer Type, 2023–2031
Figure 70: Europe Radiopharmaceutical Theranostics Market Value Share Analysis, by End-user, 2022 and 2031
Figure 71: Europe Radiopharmaceutical Theranostics Market Attractiveness Analysis, End-user, 2023–2031
Figure 72: Rest of the World Radiopharmaceutical Theranostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 73: Rest of the World Radiopharmaceutical Theranostics Market Value Share Analysis, by Radioisotope, 2022 and 2031
Figure 74: Rest of the World Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radioisotope, 2023–2031
Figure 75: Rest of the World Radiopharmaceutical Theranostics Market Value Share Analysis, by Application, 2022 and 2031
Figure 76: Rest of the World Radiopharmaceutical Theranostics Market Attractiveness Analysis, Application, 2023–2031
Figure 77: Rest of the World Radiopharmaceutical Theranostics Market Value Share Analysis, by Source, 2022 and 2031
Figure 78: Rest of the World Radiopharmaceutical Theranostics Market Attractiveness Analysis, Source, 2023–2031
Figure 79: Rest of the World Radiopharmaceutical Theranostics Market Value Share Analysis, by Approach, 2022 and 2031
Figure 80: Rest of the World Radiopharmaceutical Theranostics Market Attractiveness Analysis, Approach, 2023–2031
Figure 81: Rest of the World Radiopharmaceutical Theranostics Market Value Share Analysis, by Radiotracer Type, 2022 and 2031
Figure 82: Rest of the World Radiopharmaceutical Theranostics Market Attractiveness Analysis, Radiotracer Type, 2023–2031
Figure 83: Rest of the World Radiopharmaceutical Theranostics Market Value Share Analysis, by End-user 2022 and 2031
Figure 84: Rest of the World Radiopharmaceutical Theranostics Market Attractiveness Analysis, End-user, 2023–2031