Reports
Reports
As more is being learned about new coronavirus strains, cardiology societies have become aware about various steps needed to enable safe catheterization processes for patients and hospital personnel. Companies in the U.S. venous procedure devices market are adhering to the guidelines issued by the American College of Cardiology’s Interventional Council and the Society for Cardiovascular Angiography and Interventions.
Healthcare facilities are instituting various policies in their organizations pertaining to logistics and supply chain management. Manufacturers in the venous procedure devices market are taking into consideration these policies to prevent supply shocks. As such, delaying non-essential medical, dental, and surgical procedures is likely to affect market growth.
As the venous space continues to evolve in the U.S., manufacturers in the venous procedure devices market are learning to navigate through the challenges of the venous anatomy from their international peers. For instance, physicians find it challenging to apply the same arterial techniques in order to achieve reduced venous hypertension caused by occlusive disease.
In order to develop venous stents that improve patient outcomes, companies in the venous procedure devices market are increasing their R&D in multi-segments of diseases and evaluation of lesion types. Similarly, the Venovo™ Venous Stent is emerging as an attractive proposition to achieve compression resistance, a balance between radial strength and flexibility to address various venous challenges.
The venous procedure devices market is estimated to reach US$ 3.4 Bn by the end of 2030. Innovations in peripheral vascular interventional guidewires are contributing toward market expansion. For instance, Integer Holdings Corporation— a specialist in advanced medical device design and outsourcing, is steering design innovations in its peripheral vascular interventional guidewires for applications involving carotid, renal, and lower limb interventions.
Companies in the venous procedure devices market are increasing their portfolio in custom and standard guidewires & components, including mandrels, coils, stylets, and cores. They are increasing efforts to team up with material experts to deliver optimum product performance.
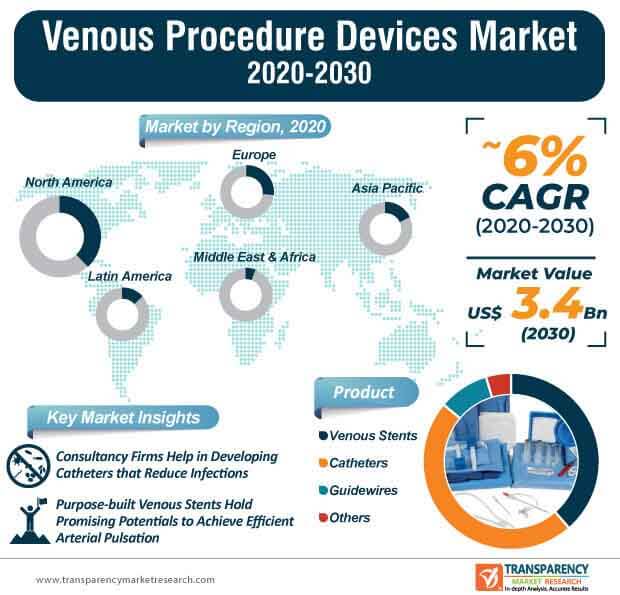
The venous procedure devices market is predicted to expand at a favorable CAGR of ~6% during the forecast period. Unmatched performance in drug eluting venous stents are contributing to market growth. For instance, Boston Scientific— a manufacturer of medical devices used in interventional medical specialties, is boosting its marketing capabilities of Eluvia drug eluting vascular stent system, which is capable of delivering exceptional outcomes in complex lesions.
The high prevalence of challenging SFA (superficial femoral artery) diseases has triggered the demand for cutting-edge drug eluting vascular stent systems. Manufacturers in the venous procedure devices market are making use of polymer designs in these stents to facilitate controlled drug release. They are gaining proficiency in randomized controlled multi-center trials to boost credibility of their products.
As increasing number of startups are tapping into incremental opportunities in the venous procedure devices market, the competition has intensified to gain licensees and acquire investors that can contribute in the development of innovative venous catheters. For instance, Team— a medical device consultancy firm is helping startups to design central venous catheters that can be comfortably attached to a patient’s skin under a sterile dressing.
Manufacturers in the venous procedure devices market are boosting their output capacities in central venous catheters that reduce the frequency of disinfection procedures and lower the risk of infections caused by tugging of the catheter. Patients needing prolonged infusion therapy are creating a demand for sterile central venous catheters.
Analysts’ Viewpoint
Since low risk patients are being differed for treatment using venous procedure devices during the ongoing coronavirus pandemic, manufacturers need to weigh the balance of staff exposure and patient benefit before planning for the upcoming financial year. Purpose-built venous stents hold promising potentials to achieve efficient arterial pulsation and mobility for the treatment of occlusion diseases. However, central venous catheters are being associated with complications such as blood clots, air embolism, and damage to blood vessels. Thus, in order to receive licenses and interest of investors for driving innovations in central venous catheters, manufacturers in the venous procedure devices market should partner with med-tech consulting firms to improve patient outcome.
Venous procedure devices market to reach valuation of US$ 3.4 Bn by 2030
Venous procedure devices market is predicted to expand at a favorable CAGR of ~6% during 2020-2030
Venous procedure devices market is driven by rise in number of patients seeking treatment and rapid adoption of new devices to treat vascular disease
North America accounted for a major share of the global venous procedure devices market, due to increase in incidence of disease and new product launches
Key players in the global venous procedures devices market include Medtronic Plc, BD, Cardinal Health, Terumo Corporation, Teleflex Incorporated, Boston Scientific Corporation
Chapter 1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
Chapter 2. Assumptions and Research Methodology
Chapter 3. Executive Summary: Global Venous Procedure Devices Market
Chapter 4. Market Overview
4.1. Introduction
4.1.1. Product Overview
4.2. Market Dynamics
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunity
Chapter 5. Key Insights
5.1. Ker Mergers and Acquisitions
5.2. Disease Overview
5.3. COVID-19 Impact Analysis
Chapter 6. Global Venous Procedure Devices Market Analysis and Forecast, by Product
6.1. Key Findings / Developments
6.2. Introduction & Definition
6.3. Global Venous Procedure Devices Market Value Forecast, by Product, 2018–2030
6.3.1. Venous Stents
6.3.1.1. Drug Eluting Venous Stent
6.3.1.2. Non-drug Eluting Venous Stent
6.3.2. Catheters
6.3.2.1. Central Venous Catheter
6.3.2.2. Subcutaneous (Implanted) Port
6.3.2.3. Peripherally Inserted Central Catheter (PICC)
6.3.3. Guidewires
6.3.3.1. Standard
6.3.3.2. High Support
6.3.3.3. Flexible
6.3.3.4. Chronic Total Occlusion
6.3.4. Others
6.4. Global Venous Procedure Devices Market Attractiveness Analysis, by Product
Chapter 7. Global Venous Procedure Devices Market Analysis and Forecast, by Application
7.1. Key Findings / Developments
7.2. Introduction & Definition
7.3. Global Venous Procedure Devices Market Value Forecast, by Application, 2018–2030
7.3.1. Leg
7.3.2. Chest
7.3.3. Abdomen
7.3.4. Arm
7.4. Global Venous Procedure Devices Market Attractiveness Analysis, by Application
Chapter 8. Global Venous Procedure Devices Market Analysis and Forecast, by Indication
8.1. Key Findings / Developments
8.2. Introduction & Definition
8.3. Global Venous Procedure Devices Market Value Forecast, by Indication, 2018–2030
8.3.1. Vascular Diseases
8.3.1.1. Chronic Deep Vein Thrombosis
8.3.1.2. Post-thrombotic Syndrome
8.3.1.3. May-Thurner Syndrome
8.3.1.4. Hemodialysis/Arteriovenous Fistulae
8.3.1.5. Others
8.3.2. Cancer
8.4. Global Venous Procedure Devices Market Attractiveness Analysis, by Indication
Chapter 9. Global Venous Procedure Devices Market Analysis and Forecast, by End-user
9.1. Key Findings / Developments
9.2. Introduction & Definition
9.3. Global Venous Procedure Devices Market Value Forecast, by End-user, 2018–2030
9.3.1. Hospitals
9.3.2. Ambulatory Surgical Centers
9.3.3. Specialty Clinics
9.3.4. Others
9.4. Global Venous Procedure Devices Market Attractiveness Analysis, by End-user
Chapter 10. Global Venous Procedure Devices Market Analysis and Forecast, by Region
10.1. Geographical Representation
10.2. Global Venous Procedure Devices Market Value Forecast, by Region
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Global Venous Procedure Devices Market Attractiveness Analysis, by Region
Chapter 11. North America Venous Procedure Devices Market Analysis and Forecast
11.1. North America Venous Procedure Devices Market Value Forecast, by Country, 2018–2030
11.1.1. U.S.
11.1.2. Canada
11.2. North America Venous Procedure Devices Market Value Forecast, by Product, 2018–2030
11.3. North America Venous Procedure Devices Market Value Forecast, by Application, 2018–2030
11.4. North America Venous Procedure Devices Market Value Forecast, by Indication, 2018–2030
11.5. North America Venous Procedure Devices Market Value Forecast, by End-user, 2018–2030
11.6. North America Venous Procedure Devices Market Attractiveness Analysis, 2020–2030
11.6.1. By Country
11.6.2. By Product
11.6.3. By Application
11.6.4. By Indication
11.6.5. By End-user
Chapter 12. Europe Venous Procedure Devices Market Analysis and Forecast
12.1. Europe Venous Procedure Devices Market Value Forecast, by Country, 2018–2030
12.1.1. Germany
12.1.2. U.K.
12.1.3. France
12.1.4. Italy
12.1.5. Spain
12.1.6. Rest of Europe
12.2. Europe Venous Procedure Devices Market Value Forecast, by Product, 2018–2030
12.3. Europe Venous Procedure Devices Market Value Forecast, by Application, 2018–2030
12.4. Europe Venous Procedure Devices Market Value Forecast, by Indication, 2018–2030
12.5. Europe Venous Procedure Devices Market Value Forecast, by End-user, 2018–2030
12.6. Europe Venous Procedure Devices Market Attractiveness Analysis, 2020–2030
12.6.1. By Country
12.6.2. By Product
12.6.3. By Application
12.6.4. By Indication
12.6.5. By End-user
Chapter 13. Asia Pacific Venous Procedure Devices Market Analysis and Forecast
13.1. Asia Pacific Venous Procedure Devices Market Value Forecast, by Country, 2018–2030
13.1.1. China
13.1.2. Japan
13.1.3. India
13.1.4. Australia & New Zealand
13.1.5. Rest of Asia Pacific
13.2. Asia Pacific Venous Procedure Devices Market Value Forecast, by Product, 2018–2030
13.3. Asia Pacific Venous Procedure Devices Market Value Forecast, by Application, 2018–2030
13.4. Asia Pacific Venous Procedure Devices Market Value Forecast, by Indication, 2018–2030
13.5. Asia Pacific Venous Procedure Devices Market Value Forecast, by End-user, 2018–2030
13.6. Asia Pacific Venous Procedure Devices Market Attractiveness Analysis, 2020–2030
13.6.1. By Country
13.6.2. By Product
13.6.3. By Application
13.6.4. By Indication
13.6.5. By End-user
Chapter 14. Latin America Venous Procedure Devices Market Analysis and Forecast
14.1. Latin America Venous Procedure Devices Market Value Forecast, by Country, 2018–2030
14.1.1. Brazil
14.1.2. Mexico
14.1.3. Rest of Latin America
14.2. Latin America Venous Procedure Devices Market Value Forecast, by Product, 2018–2030
14.3. Latin America Venous Procedure Devices Market Value Forecast, by Application, 2018–2030
14.4. Latin America Venous Procedure Devices Market Value Forecast, by Indication, 2018–2030
14.5. Latin America Venous Procedure Devices Market Value Forecast, by End-user, 2018–2030
14.6. Latin America Venous Procedure Devices Market Attractiveness Analysis, 2020–2030
14.6.1. By Country
14.6.2. By Product
14.6.3. By Application
14.6.4. By Indication
14.6.5. By End-user
Chapter 15. Middle East & Africa Venous Procedure Devices Market Analysis and Forecast
15.1. Middle East & Africa Venous Procedure Devices Market Value Forecast, by Country, 2018–2030
15.1.1. GCC Countries
15.1.2. South Africa
15.1.3. Rest of Middle East & Africa
15.2. Middle East & Africa Venous Procedure Devices Market Value Forecast, by Product, 2018–2030
15.3. Middle East & Africa Venous Procedure Devices Market Value Forecast, by Application, 2018–2030
15.4. Middle East & Africa Venous Procedure Devices Market Value Forecast, by Indication, 2018–2030
15.5. Middle East & Africa Venous Procedure Devices Market Value Forecast, by End-user, 2018–2030
15.6. Middle East & Africa Venous Procedure Devices Market Attractiveness Analysis, 2020–2030
15.6.1. By Country
15.6.2. By Product
15.6.3. By Application
15.6.4. By Indication
15.6.5. By End-user
Chapter 16. Competitive Landscape
16.1. Market Share Analysis, by Company, 2019
16.2. Company Profiles (Details - Overview, Financials, Recent Developments, Strategy)
16.2.1. Medtronic Plc
16.2.1.1. Company Details
16.2.1.2. Company Description
16.2.1.3. Business Overview
16.2.1.4. SWOT Analysis
16.2.1.5. Financial Analysis
16.2.1.6. Strategic Overview
16.2.2. Cardinal Health
16.2.2.1. Company Details
16.2.2.2. Company Description
16.2.2.3. Business Overview
16.2.2.4. SWOT Analysis
16.2.2.5. Strategic Overview
16.2.3. BD
16.2.3.1. Company Details
16.2.3.2. Company Description
16.2.3.3. Business Overview
16.2.3.4. SWOT Analysis
16.2.3.5. Strategic Overview
16.2.4. Terumo Corporation
16.2.4.1. Company Details
16.2.4.2. Company Description
16.2.4.3. Business Overview
16.2.4.4. SWOT Analysis
16.2.4.5. Strategic Overview
16.2.5. Teleflex Incorporated
16.2.5.1. Company Details
16.2.5.2. Company Description
16.2.5.3. Business Overview
16.2.5.4. SWOT Analysis
16.2.5.5. Financial Analysis
16.2.5.6. Strategic Overview
16.2.6. Boston Scientific Corporation
16.2.6.1. Company Details
16.2.6.2. Company Description
16.2.6.3. Business Overview
16.2.6.4. SWOT Analysis
16.2.6.5. Financial Analysis
16.2.6.6. Strategic Overview
16.2.7. Optimed Medizinische Instrumente GmbH
16.2.7.1. Company Details
16.2.7.2. Company Description
16.2.7.3. Business Overview
16.2.7.4. SWOT Analysis
16.2.7.5. Financial Analysis
16.2.7.6. Strategic Overview
16.2.8. Cook
16.2.8.1. Company Details
16.2.8.2. Company Description
16.2.8.3. Business Overview
16.2.8.4. SWOT Analysis
16.2.8.5. Financial Analysis
16.2.8.6. Strategic Overview
16.2.9. B. Braun Melsungen AG
16.2.9.1. Company Details
16.2.9.2. Company Description
16.2.9.3. Business Overview
16.2.9.4. SWOT Analysis
16.2.9.5. Financial Analysis
16.2.9.6. Strategic Overview
16.2.10. Abbott
16.2.10.1. Company Details
16.2.10.2. Company Description
16.2.10.3. Business Overview
16.2.10.4. SWOT Analysis
16.2.10.5. Financial Analysis
16.2.10.6. Strategic Overview
List of Tables
Table 01: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 02: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Venous Stents, 2018–2030
Table 03: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Catheters, 2018–2030
Table 04: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Guidewires, 2018–2030
Table 05: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Application, 2018–2030
Table 06: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 07: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Vascular Diseases, 2018–2030
Table 08: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by End-user, 2018–2030
Table 09: Global Venous Procedures Devices Market Value (US$ Mn) Forecast, by Region, 2018–2030
Table 10: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Country, 2018–2030
Table 11: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 12: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Venous Stents, 2018–2030
Table 13: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Catheters, 2018–2030
Table 14: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 15: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Application, 2018–2030
Table 16: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 17: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Vascular Diseases, 2018–2030
Table 18: North America Venous Procedures Devices Market Value (US$ Mn) Forecast, by End-user, 2018–2030
Table 19: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Countries/Sub-region, 2018–2030
Table 20: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 21: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Venous Stents, 2018–2030
Table 22: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Catheters, 2018–2030
Table 23: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 24: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Application, 2018–2030
Table 25: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 26: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by Vascular Diseases, 2018–2030
Table 27: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast, by End-user, 2018–2030
Table 28: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Countries/Sub-region, 2018–2030
Table 29: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 30: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Venous Stents, 2018–2030
Table 31: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Catheters, 2018–2030
Table 32: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 33: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Application, 2018–2030
Table 34: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 35: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by Vascular Diseases, 2018–2030
Table 36: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast, by End-user, 2018–2030
Table 37: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Countries/Sub-region, 2018–2030
Table 38: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 39: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Venous Stents, 2018–2030
Table 40: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Catheters, 2018–2030
Table 41: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Guidewires, 2018–2030
Table 42: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Application, 2018–2030
Table 43: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 44: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by Vascular Diseases, 2018–2030
Table 45: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast, by End-user, 2018–2030
Table 46: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Countries/Sub-region, 2018–2030
Table 47: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Product, 2018–2030
Table 48: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Venous Stents, 2018–2030
Table 49: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Catheters, 2018–2030
Table 50: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Guidewires, 2018–2030
Table 51: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Application, 2018–2030
Table 52: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 53: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by Vascular Diseases, 2018–2030
Table 54: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast, by End-user, 2018–2030
List of Figures
Figure 01: Global Venous Procedure Devices Market Value (US$ Mn) Forecast, 2018–2030
Figure 02: Global Venous Procedure Devices Market Value Share, by Product (2019)
Figure 03: Global Venous Procedure Devices Market Value Share, by Application (2019)
Figure 04: Global Venous Procedure Devices Market Value Share, by Indication (2019)
Figure 05: Global Venous Procedure Devices Market Value Share, by End-user (2019)
Figure 06: Global Venous Procedure Devices Market Value Share, by Region (2019)
Figure 07: Global Venous Procedures Devices Market Value Share Analysis, by Product, 2019 and 2030
Figure 08: Global Venous Procedures Devices Market Value (US$ Mn), by Venous Stents, 2018–2030
Figure 09: Global Venous Procedures Devices Market Value (US$ Mn), by Catheters, 2018–2030
Figure 10: Global Venous Procedures Devices Market Value (US$ Mn), by Guidewires, 2018–2030
Figure 11: Global Venous Procedures Devices Market Value (US$ Mn), by Others, 2018–2030
Figure 12: Global Venous Procedures Devices Market Attractiveness Analysis, by Product, 2020–2030
Figure 13: Global Venous Procedures Devices Market Value Share Analysis, by Application, 2019 and 2030
Figure 14: Global Venous Procedures Devices Market Value (US$ Mn), by Leg, 2018–2030
Figure 15: Global Venous Procedures Devices Market Value (US$ Mn), by Chest, 2018–2030
Figure 16: Global Venous Procedures Devices Market Value (US$ Mn), by Abdomen, 2018–2030
Figure 17: Global Venous Procedures Devices Market Value (US$ Mn), by Arm, 2018–2030
Figure 18: Global Venous Procedures Devices Market Attractiveness Analysis, by Application, 2020–2030
Figure 19: Global Venous Procedures Devices Market Value Share Analysis, by Indication, 2019 and 2030
Figure 20: Global Venous Procedures Devices Market Value (US$ Mn), by Vascular Diseases, 2018–2030
Figure 21: Global Venous Procedures Devices Market Value (US$ Mn), by Cancer, 2018–2030
Figure 22: Global Venous Procedures Devices Market Attractiveness Analysis, by Indication, 2020–2030
Figure 23: Global Venous Procedures Devices Market Value Share Analysis, by End-user, 2019 and 2030
Figure 24: Global Venous Procedures Devices Market Value (US$ Mn), by Hospitals, 2018–2030
Figure 25: Global Venous Procedures Devices Market Value (US$ Mn), by Specialty Clinics, 2018–2030
Figure 26: Global Venous Procedures Devices Market Value (US$ Mn), by Ambulatory Surgical Centers, 2018–2030
Figure 27: Global Venous Procedures Devices Market Value (US$ Mn), by Others, 2018–2030
Figure 28: Global Venous Procedures Devices Market Attractiveness Analysis, by End-user, 2020–2030
Figure 29: Global Venous Procedures Devices Market Value Share Analysis, by Region, 2019 and 2030
Figure 30: Global Venous Procedures Devices Market Analysis, by Region
Figure 31: North America Venous Procedures Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 32: North America Venous Procedures Devices Market Value Share (%), by Country, 2019 and 2030
Figure 33: North America Venous Procedures Devices Market Attractiveness, by Country, 2020–2030
Figure 34: North America Venous Procedures Devices Market Value Share Analysis, by Product, 2019 and 2030
Figure 35: North America Venous Procedures Devices Market Attractiveness, by Product, 2020–2030
Figure 36: North America Venous Procedures Devices Market Value Share Analysis, by Application, 2019 and 2030
Figure 37: North America Venous Procedures Devices Market Attractiveness, by Application, 2020–2030
Figure 38: North America Venous Procedures Devices Market Value Share Analysis, by Indication, 2019 and 2030
Figure 39: North America Venous Procedures Devices Market Attractiveness, by Indication, 2020–2030
Figure 40: North America Venous Procedures Devices Market Value Share Analysis, by End-user, 2019 and 2030
Figure 41: North America Venous Procedures Devices Market Attractiveness, by End-user, 2020–2030
Figure 42: Europe Venous Procedures Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 43: Europe Venous Procedures Devices Market Value Share (%), by Countries/Sub-region, 2019 and 2030
Figure 44: Europe Venous Procedures Devices Market Attractiveness, by Countries/Sub-region, 2020–2030
Figure 45: Europe Venous Procedures Devices Market Value Share Analysis, by Product, 2019 and 2030
Figure 46: Europe Venous Procedures Devices Market Attractiveness, by Product, 2020–2030
Figure 47: Europe Venous Procedures Devices Market Value Share Analysis, by Application, 2019 and 2030
Figure 48: Europe Venous Procedures Devices Market Attractiveness, by Application, 2020–2030
Figure 49: Europe Venous Procedures Devices Market Value Share Analysis, by Indication, 2019 and 2030
Figure 50: Europe Venous Procedures Devices Market Attractiveness, by Indication, 2020–2030
Figure 51: Europe Venous Procedures Devices Market Value Share Analysis, by End-user, 2019 and 2030
Figure 52: Europe Venous Procedures Devices Market Attractiveness, by End-user, 2020–2030
Figure 53: Asia Pacific Venous Procedures Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 54: Asia Pacific Venous Procedures Devices Market Value Share (%), by Countries/Sub-region, 2019 and 2030
Figure 55: Asia Pacific Venous Procedures Devices Market Attractiveness, by Countries/Sub-region, 2020–2030
Figure 56: Asia Pacific Venous Procedures Devices Market Value Share Analysis, by Product, 2019 and 2030
Figure 57: Asia Pacific Venous Procedures Devices Market Attractiveness, by Product, 2020–2030
Figure 58: Asia Pacific Venous Procedures Devices Market Value Share Analysis, by Application, 2019 and 2030
Figure 59: Asia Pacific Venous Procedures Devices Market Attractiveness, by Application, 2020–2030
Figure 60: Asia Pacific Venous Procedures Devices Market Value Share Analysis, by Indication, 2019 and 2030
Figure 61: Asia Pacific Venous Procedures Devices Market Attractiveness, by Indication, 2020–2030
Figure 61: Asia Pacific Venous Procedures Devices Market Value Share Analysis, by End-user, 2019 and 2030
Figure 63: Asia Pacific Venous Procedures Devices Market Attractiveness, by End-user, 2020–2030
Figure 64: Latin America Venous Procedures Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 65: Latin America Venous Procedures Devices Market Value Share (%), by Countries/Sub-region, 2019 and 2030
Figure 66: Latin America Venous Procedures Devices Market Attractiveness Analysis, by Countries/Sub-region, 2020–2030
Figure 67: Latin America Venous Procedures Devices Market Value Share Analysis, by Product, 2019 and 2030
Figure 68: Latin America Venous Procedures Devices Market Attractiveness Analysis, by Product, 2020–2030
Figure 69: Latin America Venous Procedures Devices Market Value Share Analysis, by Application, 2019 and 2030
Figure 70: Latin America Venous Procedures Devices Market Attractiveness Analysis, by Application, 2020–2030
Figure 71: Latin America Venous Procedures Devices Market Value Share Analysis, by Indication, 2019 and 2030
Figure 72: Latin America Venous Procedures Devices Market Attractiveness Analysis, by Indication, 2020–2030
Figure 73: Latin America Venous Procedures Devices Market Value Share Analysis, by End-user, 2019 and 2030
Figure 74: Latin America Venous Procedures Devices Market Attractiveness Analysis, by End-user, 2020–2030
Figure 75: Middle East & Africa Venous Procedures Devices Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 76: Middle East & Africa Venous Procedures Devices Market Value Share (%), by Countries/Sub-region, 2019 and 2030
Figure 77: Middle East & Africa Venous Procedures Devices Market Attractiveness Analysis, by Countries/Sub-region, 2020–2030
Figure 78: Middle East & Africa Venous Procedures Devices Market Value Share Analysis, by Product, 2019 and 2030
Figure 79: Middle East & Africa Venous Procedures Devices Market Attractiveness Analysis, by Product, 2020–2030
Figure 80: Middle East & Africa Venous Procedures Devices Market Value Share Analysis, by Application, 2019 and 2030
Figure 81: Middle East & Africa Venous Procedures Devices Market Attractiveness Analysis, by Application, 2020–2030
Figure 82: Middle East & Africa Venous Procedures Devices Market Value Share Analysis, by Indication, 2019 and 2030
Figure 83: Middle East & Africa Venous Procedures Devices Market Attractiveness Analysis, by Indication, 2020–2030
Figure 84: Middle East & Africa Venous Procedures Devices Market Value Share Analysis, by End-user, 2019 and 2030
Figure 85: Middle East & Africa Venous Procedures Devices Market Attractiveness Analysis, by End-user, 2020–2030