Reports
Reports
The ever-evolving nature of coronavirus has become a major threat to the public health, owing to its severe respiratory symptoms. Ongoing studies are surfacing about the safety and efficacy of novel lyophilized therapeutic agents in hospitalized adult patients diagnosed with COVID-19. Amid all this, healthcare giant Cipla - a global pharmaceutical company is gaining recognition for introducing its CIPREMI, a remdesivir lyophilized powder for injection (100mg). Manufacturers in the lyophilized injectable market are taking cues from such innovations to increase the availability of the U.S. FDA-approved emergency use authorization (EUA) treatment for patients with severe COVID-19 symptoms.
Since India is suffering from a third wave of the pandemic, companies in the India lyophilized injectable market are increasing efforts to gain regulatory approval by the Drug Controller General of India (DCGI) for restricted emergency use in the country.
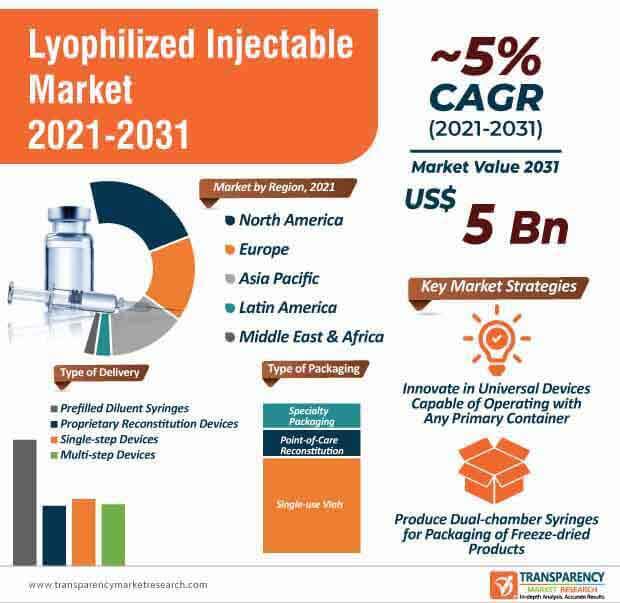
The lyophilized injectable market is expected to surpass US$ 5 Bn by 2031. Packaging plays a crucial role in the lyophilization of injectable drugs. Though lyo-breakage is generally not a routine occurrence, however, even at a low frequency, it can lead to high cost and operational impacts. Hence, companies are adopting alternative vial designs to reduce lyo-breakage risk and reduce significant side-load force during vial transfer in/out of the lyophilizer.
Since lyophilization is a demanding process performed with costly equipment, manufacturers are reducing significant vertical-load forces during stopper closure to avoid lyo-breakage.
The lyophilized injectable market is estimated to clock a CAGR of ~5% during the forecast period. However, many biotechnologically manufactured substances are not sufficiently stable in aqueous solutions. This becomes challenging to preserve liquid formulations for longer periods. In order to overcome this, companies are adopting freeze-drying by which water is extracted from the substance under vacuum and low temperatures. There is a growing awareness to reconstitute drugs before administering them to patients.
Companies in the lyophilized injectable market are bolstering their production capabilities in freeze-dried substances, which are being packaged in vials, dual-chamber syringes, and dual-chamber cartridges. Vials have set the gold standard for freeze-dried products.
Universal, customizable, and upgradable drug devices are anticipated to help companies in the lyophilized injectable market gain a competitive edge over other market players during the forecast period. Manufacturers are boosting their R&D to develop devices and injectors that are universal in nature and capable of operating with the help of any primary container, sans the need for a drug transfer step.
Embedded electronic features in devices and injectors are predicted to emerge as a game changer for stakeholders in the global lyophilized injectable market. These features will allow customization depending on the intended user type. Other connectivity capabilities will enable devices to be remotely upgraded, whilst making the task of lifecycle management easier.
As companies in the lyophilized injectable market are gaining awareness about the exact causes of lyo-breakage, they are increasing their production capabilities in stronger vials that may lead to a reduced likelihood of occurrence. SGD Pharma - a specialist in the production of pharmaceutical glass primary packaging has introduced its EasyLyo vial range, which combines the strength and chemical durability of molded vials with characteristics of thin-walled vials.
Manufacturers in the lyophilized injectable market are increasing efforts to reduce breakage rates and heat-transfer capability in vials. They are conducting several lyo-breakage tests using worst-case injectable formulations and cycle designed to break vials. Companies are producing flat base vials with squared-off shoulder and heel vials to yield better performance in lyophilization applications.
Analysts’ Viewpoint
Companies in the lyophilized injectable market are anticipated to enter into partnerships to increase accessibility to remdesivir for patients suffering from COVID-19 in India. Injectable devices are expected to align with the increased requirement of greener technologies. However, lyo-breakage is emerging as a major issue in packaging applications. Hence, companies should develop vials with improved uniformity and cosmetic nature to offer better inspectability & performance as compared to standard molded vials. They are developing injectors that will allow patients to take control of their disease by monitoring their drug performance. Dual-chamber syringes are one of the most innovative packaging forms for freeze-dried products.
Lyophilized injectable market is expected to surpass US$ 5 Bn by 2031
Lyophilized injectable market is projected to expand at a CAGR of ~5% from 2021 to 2031
Lyophilized injectable market is driven by rapidly growing contract research manufacturing services (CRAMS) and expansion of pipeline of lyophilized injectable drugs
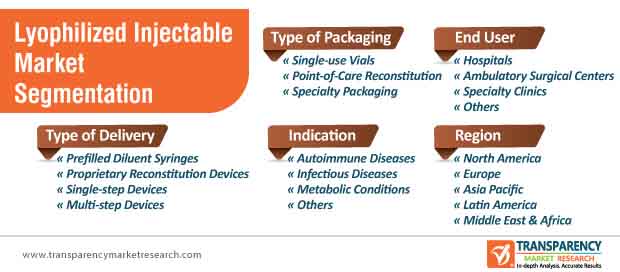
The end-use segments in lyophilized injectable market are hospitals, ambulatory surgical centers and specialty clinics
Key players in the global lyophilized injectable market include B. Braun Melsungen AG, Baxter International, Inc., BD, Schott AG, Aristopharma Ltd., Vetter Pharma, and Jubilant HollisterStier LLC.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Lyophilized Injectable Market
4. Market Overview
4.1. Introduction & Overview
4.2. Market Dynamics
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunities
4.3. Global Lyophilized Injectable Market Analysis and Forecast, 2017–2031
5. Market Outlook
5.1. COVID-19 Pandemics Impact on Industry
5.2. Key Industry Developments
5.3. Pipeline Analysis
5.4. Top 10 Lyophilized Drug Products
5.5. Regulatory Scenario
6. Global Lyophilized Injectable Market Analysis and Forecast, by Type of Packaging
6.1. Introduction & Definition
6.2. Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
6.2.1. Single-use Vials
6.2.2. Point-of-care Reconstitution
6.2.3. Specialty Packaging
6.3. Global Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging
7. Global Lyophilized Injectable Market Analysis and Forecast, by Type of Delivery
7.1. Introduction & Definition
7.2. Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
7.2.1. Prefilled Diluent Syringes
7.2.2. Proprietary Reconstitution Devices
7.2.3. Single-step Devices
7.2.4. Multi-step Devices
7.3. Global Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery
8. Global Lyophilized Injectable Market Analysis and Forecast, by Indication
8.1. Introduction & Definition
8.2. Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
8.2.1. Autoimmune Diseases
8.2.2. Metabolic Conditions
8.2.3. Infectious Diseases
8.2.4. Others
8.3. Global Lyophilized Injectable Market Attractiveness Analysis, by Indication
9. Global Lyophilized Injectable Market Analysis and Forecast, by End-user
9.1. Introduction & Definition
9.2. Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
9.2.1. Hospitals
9.2.2. Ambulatory Surgical Centers
9.2.3. Specialty Clinics
9.2.4. Others
9.3. Global Lyophilized Injectable Market Attractiveness Analysis, by End-user
10. Global Lyophilized Injectable Market Analysis and Forecast, by Region
10.1. Key Findings
10.2. Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Region
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Global Lyophilized Injectable Market Attractiveness Analysis, by Region
11. North America Lyophilized Injectable Market Analysis and Forecast
11.1. Introduction
11.2. North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
11.2.1. Single-use Vials
11.2.2. Point-of-care Reconstitution
11.2.3. Specialty Packaging
11.3. North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
11.3.1. Prefilled Diluent Syringes
11.3.2. Proprietary Reconstitution Devices
11.3.3. Single-step Devices
11.3.4. Multi-step Devices
11.4. North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
11.4.1. Autoimmune Diseases
11.4.2. Metabolic Conditions
11.4.3. Infectious Diseases
11.4.4. Others
11.5. North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
11.5.1. Hospitals
11.5.2. Ambulatory Surgical Centers
11.5.3. Specialty Clinics
11.5.4. Others
11.6. North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country, 2017–2031
11.6.1. U.S.
11.6.2. Canada
11.7. North America Lyophilized Injectable Market Attractiveness Analysis
11.7.1. By Type of Packaging
11.7.2. By Type of Delivery
11.7.3. By Indication
11.7.4. By End-user
11.7.5. By Country
12. Europe Lyophilized Injectable Market Analysis and Forecast
12.1. Introduction
12.2. Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
12.2.1. Single-use Vials
12.2.2. Point-of-care Reconstitution
12.2.3. Specialty Packaging
12.3. Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
12.3.1. Prefilled Diluent Syringes
12.3.2. Proprietary Reconstitution Devices
12.3.3. Single-step Devices
12.3.4. Multi-step Devices
12.4. Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
12.4.1. Autoimmune Diseases
12.4.2. Metabolic Conditions
12.4.3. Infectious Diseases
12.4.4. Others
12.5. Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
12.5.1. Hospitals
12.5.2. Ambulatory Surgical Centers
12.5.3. Specialty Clinics
12.5.4. Others
12.6. Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
12.6.1. Germany
12.6.2. U.K.
12.6.3. France
12.6.4. Spain
12.6.5. Italy
12.6.6. Rest of Europe
12.7. Europe Lyophilized Injectable Market Attractiveness Analysis
12.7.1. By Type of Packaging
12.7.2. By Type of Delivery
12.7.3. By Indication
12.7.4. By End-user
12.7.5. By Country/Sub-region
13. Asia Pacific Lyophilized Injectable Market Analysis and Forecast
13.1. Introduction
13.2. Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
13.2.1. Single-use Vials
13.2.2. Point-of-care Reconstitution
13.2.3. Specialty Packaging
13.3. Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
13.3.1. Prefilled Diluent Syringes
13.3.2. Proprietary Reconstitution Devices
13.3.3. Single-step Devices
13.3.4. Multi-step Devices
13.4. Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
13.4.1. Autoimmune Diseases
13.4.2. Metabolic Conditions
13.4.3. Infectious Diseases
13.4.4. Others
13.5. Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
13.5.1. Hospitals
13.5.2. Ambulatory Surgical Centers
13.5.3. Specialty Clinics
13.5.4. Others
13.6. Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
13.6.1. China
13.6.2. Japan
13.6.3. India
13.6.4. Australia & New Zealand
13.6.5. Rest of Asia Pacific
13.7. Asia Pacific Lyophilized Injectable Market Attractiveness Analysis
13.7.1. By Type of Packaging
13.7.2. By Type of Delivery
13.7.3. By Indication
13.7.4. By End-user
13.7.5. By Country/Sub-region
14. Latin America Lyophilized Injectable Market Analysis and Forecast
14.1. Introduction
14.2. Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
14.2.1. Single-use Vials
14.2.2. Point-of-care Reconstitution
14.2.3. Specialty Packaging
14.3. Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
14.3.1. Prefilled Diluent Syringes
14.3.2. Proprietary Reconstitution Devices
14.3.3. Single-step Devices
14.3.4. Multi-step Devices
14.4. Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
14.4.1. Autoimmune Diseases
14.4.2. Metabolic Conditions
14.4.3. Infectious Diseases
14.4.4. Others
14.5. Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
14.5.1. Hospitals
14.5.2. Ambulatory Surgical Centers
14.5.3. Specialty Clinics
14.5.4. Others
14.6. Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
14.6.1. Brazil
14.6.2. Mexico
14.6.3. Rest of Latin America
14.7. Latin America Lyophilized Injectable Market Attractiveness Analysis
14.7.1. By Type of Packaging
14.7.2. By Type of Delivery
14.7.3. By Indication
14.7.4. By End-user
14.7.5. By Country/Sub-region
15. Middle East & Africa Lyophilized Injectable Market Analysis and Forecast
15.1. Introduction
15.2. Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
15.2.1. Single-use Vials
15.2.2. Point-of-care Reconstitution
15.2.3. Specialty Packaging
15.3. Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
15.3.1. Prefilled Diluent Syringes
15.3.2. Proprietary Reconstitution Devices
15.3.3. Single-step Devices
15.3.4. Multi-step Devices
15.4. Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
15.4.1. Autoimmune Diseases
15.4.2. Metabolic Conditions
15.4.3. Infectious Diseases
15.4.4. Others
15.5. Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
15.5.1. Hospitals
15.5.2. Ambulatory Surgical Centers
15.5.3. Specialty Clinics
15.5.4. Others
15.6. Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
15.6.1. GCC Countries
15.6.2. South Africa
15.6.3. Rest of Middle East & Africa
15.7. Middle East & Africa Lyophilized Injectable Market Attractiveness Analysis
15.7.1. By Type of Packaging
15.7.2. By Type of Delivery
15.7.3. By Indication
15.7.4. By End-user
15.7.5. By Country/Sub-region
16. Competition Landscape
16.1. Market Position Analysis, by Company, 2019
16.2. Company Profiles
16.2.1. B. Braun Melsungen AG
16.2.1.1. Company Overview (HQ, Business Segments, Employee Strength)
16.2.1.2. Growth Strategies
16.2.1.3. SWOT Analysis
16.2.2. Baxter International, Inc.
16.2.2.1. Company Overview (HQ, Business Segments, Employee Strength)
16.2.2.2. Growth Strategies
16.2.2.3. SWOT Analysis
16.2.3. BD
16.2.3.1. Company Overview (HQ, Business Segments)
16.2.3.2. Growth Strategies
16.2.3.3. SWOT Analysis
16.2.4. SCHOTT AG
16.2.4.1. Company Overview (HQ, Business Segments, Employee Strength)
16.2.4.2. Growth Strategies
16.2.4.3. SWOT Analysis
16.2.5. Aristopharma Ltd.
16.2.5.1. Company Overview (HQ, Business Segments)
16.2.5.2. Growth Strategies
16.2.5.3. SWOT Analysis
16.2.6. Vetter Pharma
16.2.6.1. Company Overview (HQ, Business Segments, Employee Strength)
16.2.6.2. Growth Strategies
16.2.6.3. SWOT Analysis
16.2.7. Jubilant HollisterStier LLC
16.2.7.1. Company Overview (HQ, Business Segments, Employee Strength)
16.2.7.2. Growth Strategies
16.2.7.3. SWOT Analysis
List of Tables
Table 01: Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
Table 02: Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
Table 03: Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 04: Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 05: Global Lyophilized Injectable Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 06: North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 07: North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
Table 08: North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
Table 09: North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 10: North America Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 11: Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 12: Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
Table 13: Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
Table 14: Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 15: Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 16: Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 17: Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
Table 18: Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
Table 19: Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 20: Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 21: Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 22: Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
Table 23: Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
Table 24: Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 25: Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 26: Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 27: Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Packaging, 2017–2031
Table 28: Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Type of Delivery, 2017–2031
Table 29: Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 30: Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, by End-user, 2017–2031
List of Figures
Figure 01: Global Lyophilized Injectable Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 02: Global Lyophilized Injectable Market Value Share, by Region, 2020
Figure 03: Global Lyophilized Injectable Market Value Share Analysis, by Type of Packaging, 2020 and 2031
Figure 04: Global Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging, 2021–2031
Figure 05: Global Lyophilized Injectable Market Value (US$ Mn), by Single-use Vials, 2017–2031
Figure 06: Global Lyophilized Injectable Market Value (US$ Mn), by Point-of-care Reconstitution, 2017–2031
Figure 07: Global Lyophilized Injectable Market Value (US$ Mn), by Specialty Packaging, 2017–2031
Figure 08: Global Lyophilized Injectable Market Value Share Analysis, by Type of Delivery, 2020 and 2031
Figure 09: Global Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery, 2021–2031
Figure 10: Global Lyophilized Injectable Market Value (US$ Mn), by Prefilled Diluent Syringes, 2017–2031
Figure 11: Global Lyophilized Injectable Market Value (US$ Mn), by Proprietary Reconstitution Devices, 2017–2031
Figure 12: Global Lyophilized Injectable Market Value (US$ Mn), by Single-step devices, 2017–2031
Figure 13: Global Lyophilized Injectable Market Value (US$ Mn), by Multi-step devices, 2017–2031
Figure 14: Global Lyophilized Injectable Market Value Share Analysis, by Indication, 2020 and 2031
Figure 15: Global Lyophilized Injectable Market Attractiveness Analysis, by Indication, 2021–2031
Figure 16: Global Lyophilized Injectable Market Value (US$ Mn), by Autoimmune Diseases, 2017–2031
Figure 17: Global Lyophilized Injectable Market Value (US$ Mn), by Infectious Diseases, 2017–2031
Figure 18: Global Lyophilized Injectable Market Value (US$ Mn), by Metabolic Conditions, 2017–2031
Figure 19: Global Lyophilized Injectable Market Value (US$ Mn), by Others, 2017–2031
Figure 20: Global Lyophilized Injectable Market Value Share Analysis, by End-user, 2020 and 2031
Figure 21: Global Lyophilized Injectable Market Attractiveness Analysis, by End-user, 2021–2031
Figure 22: Global Lyophilized Injectable Market Value (US$ Mn), by Hospitals, 2017–2031
Figure 23: Global Lyophilized Injectable Market Value (US$ Mn), by Ambulatory Surgical Centers, 2017–2031
Figure 24: Global Lyophilized Injectable Market Value (US$ Mn), by Specialty Clinics, 2017–2031
Figure 25: Global Lyophilized Injectable Market Value (US$ Mn), by Others, 2017–2031
Figure 26: Global Lyophilized Injectable Market Value Share Analysis, by Region, 2020 and 2031
Figure 27: Global Lyophilized Injectable Market Attractiveness Analysis, by Region, 2021–2031
Figure 28: North America Lyophilized Injectable Market Value (US$ Mn) Forecast, 2017–2031
Figure 29: North America Lyophilized Injectable Market Value Share Analysis, by Country, 2020 and 2031
Figure 30: North America Lyophilized Injectable Market Attractiveness Analysis, by Country, 2021–2031
Figure 31: North America Lyophilized Injectable Market Value Share Analysis, by Type of Packaging, 2020 and 2031
Figure 32: North America Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging, 2021–2031
Figure 33: North America Lyophilized Injectable Market Value Share Analysis, by Type of Delivery, 2020 and 2031
Figure 34: North America Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery, 2021–2031
Figure 35: North America Lyophilized Injectable Market Value Share Analysis, by Indication, 2020 and 2031
Figure 36: North America Lyophilized Injectable Market Attractiveness Analysis, by Indication, 2021–2031
Figure 37: North America Lyophilized Injectable Market Value Share Analysis, by End-user, 2020 and 2031
Figure 38: North America Lyophilized Injectable Market Attractiveness Analysis, by End-user, 2021–2031
Figure 39: Europe Lyophilized Injectable Market Value (US$ Mn) Forecast, 2017–2031
Figure 40: Europe Lyophilized Injectable Market Value Share Analysis, by Country/Sub-region, 2020 and 2031
Figure 41: Europe Lyophilized Injectable Market Attractiveness Analysis, by Country/Sub-region, 2021–2031
Figure 42: Europe Lyophilized Injectable Market Value Share Analysis, by Type of Packaging, 2020 and 2031
Figure 43: Europe Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging, 2021–2031
Figure 44: Europe Lyophilized Injectable Market Value Share Analysis, by Type of Delivery, 2020 and 2031
Figure 45: Europe Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery, 2021–2031
Figure 46: Europe Lyophilized Injectable Market Value Share Analysis, by Indication, 2020 and 2031
Figure 47: Europe Lyophilized Injectable Market Attractiveness Analysis, by Indication, 2021–2031
Figure 48: Europe Lyophilized Injectable Market Value Share Analysis, by End-user, 2020 and 2031
Figure 49: Europe Lyophilized Injectable Market Attractiveness Analysis, by End-user, 2021–2031
Figure 50: Asia Pacific Lyophilized Injectable Market Value (US$ Mn) Forecast, 2017–2031
Figure 51: Asia Pacific Lyophilized Injectable Market Value Share Analysis, by Country/Sub-region, 2020 and 2031
Figure 52: Asia Pacific Lyophilized Injectable Market Attractiveness Analysis, by Country/Sub-region, 2021–2031
Figure 53: Asia Pacific Lyophilized Injectable Market Value Share Analysis, by Type of Packaging, 2020 and 2031
Figure 54: Asia Pacific Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging, 2021–2031
Figure 55: Asia Pacific Lyophilized Injectable Market Value Share Analysis, by Type of Delivery, 2020 and 2031
Figure 56: Asia Pacific Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery, 2021–2031
Figure 57: Asia Pacific Lyophilized Injectable Market Value Share Analysis, by Indication, 2020 and 2031
Figure 58: Asia Pacific Lyophilized Injectable Market Attractiveness Analysis, by Indication, 2021–2031
Figure 59: Asia Pacific Lyophilized Injectable Market Value Share Analysis, by End-user, 2020 and 2031
Figure 60: Asia Pacific Lyophilized Injectable Market Attractiveness Analysis, by End-user, 2021–2031
Figure 61: Latin America Lyophilized Injectable Market Value (US$ Mn) Forecast, 2017–2031
Figure 62: Latin America Lyophilized Injectable Market Value Share Analysis, by Country, 2020 and 2031
Figure 63: Latin America Lyophilized Injectable Market Attractiveness Analysis, by Country, 2021–2031
Figure 64: Latin America Lyophilized Injectable Market Value Share Analysis, by Type of Packaging, 2020 and 2031
Figure 65: Latin America Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging, 2021–2031
Figure 66: Latin America Lyophilized Injectable Market Value Share Analysis, by Type of Delivery, 2020 and 2031
Figure 67: Latin America Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery, 2021–2031
Figure 68: Latin America Lyophilized Injectable Market Value Share Analysis, by Indication, 2020 and 2031
Figure 69: Latin America Lyophilized Injectable Market Attractiveness Analysis, by Indication, 2021–2031
Figure 70: Latin America Lyophilized Injectable Market Value Share Analysis, by End-user, 2020 and 2031
Figure 71: Latin America Lyophilized Injectable Market Attractiveness Analysis, by End-user, 2021–2031
Figure 72: Middle East & Africa Lyophilized Injectable Market Value (US$ Mn) Forecast, 2017–2031
Figure 73: Middle East & Africa Lyophilized Injectable Market Value Share Analysis, by Country/Sub-region, 2020 and 2031
Figure 74: Middle East & Africa Lyophilized Injectable Market Attractiveness Analysis, by Country/Sub-region, 2021–2031
Figure 75: Middle East & Africa Lyophilized Injectable Market Value Share Analysis, by Type of Packaging, 2020 and 2031
Figure 76: Middle East & Africa Lyophilized Injectable Market Attractiveness Analysis, by Type of Packaging, 2021–2031
Figure 77: Middle East & Africa Lyophilized Injectable Market Value Share Analysis, by Type of Delivery, 2020 and 2031
Figure 78: Middle East & Africa Lyophilized Injectable Market Attractiveness Analysis, by Type of Delivery, 2021–2031
Figure 79: Middle East & Africa Lyophilized Injectable Market Value Share Analysis, by Indication, 2020 and 2031
Figure 80: Middle East & Africa Lyophilized Injectable Market Attractiveness Analysis, by Indication, 2021–2031
Figure 81: Middle East & Africa Lyophilized Injectable Market Value Share Analysis, by End-user, 2020 and 2031
Figure 82: Middle East & Africa Lyophilized Injectable Market Attractiveness Analysis, by End-user, 2021–2031