Reports
Reports
Analysts’ Viewpoint
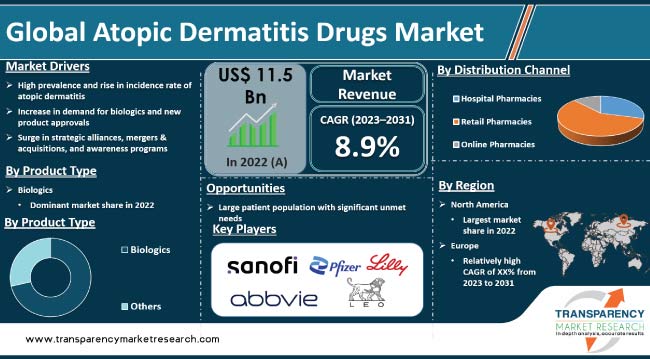
Rise in prevalence of atopic dermatitis is driving the global atopic dermatitis drugs market. Prevalence of atopic dermatitis has been increasing, with a significant rise in both adults and children. Introduction of innovative therapies and treatment options for atopic dermatitis are expected to propel market expansion. Furthermore, increase in demand for biologics, rise in new product approvals, and surge in healthcare expenditure, especially in developing countries, are likely to bolster the global atopic dermatitis drugs market size during the forecast period.
Development of effective and targeted therapies for atopic dermatitis offers lucrative opportunities to market players. Leading companies are focusing on improving access to healthcare services and providing affordable treatments such as targeted biologic therapies and topical medications in order to increase market share.
Atopic dermatitis is a skin condition that causes dry, itchy, and inflamed skin. It is most common in young children, but it could happen at any age. Atopic dermatitis is a chronic condition that flares up from time to time. It can be annoying, but it is not contagious.
Atopic dermatitis is a common disease that typically manifests itself during infancy and childhood. Several children's atopic dermatitis resolves before adolescence. Some children with atopic dermatitis may continue to have symptoms as teenagers and adults. In some cases, the disease manifests itself during adulthood.
Atopic dermatitis has shown positive response to biologic therapies. These therapies have improved the overall quality of life. Hence, demand for biologic drugs for atopic dermatitis has increased significantly, and the trend is likely to continue in the next few years.
Dermatological disorders differ across the globe based on geographic location, climatic conditions, socioeconomic status, lifestyles, age, gender, heredity, and personal habits. High prevalence of atopic dermatitis across the globe is a major factor driving the atopic dermatitis drugs market growth.
According to the World Health Organization (WHO), over 900 million people were affected with skin disease in 2017, and around 80% of the skin disorders are accounted for by the five common skin disorders. Atopic dermatitis, a common and chronic inflammatory skin disease, affects 15% to 20% of children and 1% to 3% of adults across the world.
In 2022, atopic dermatitis affected 223 million people globally, of these 43 million were children under the age of five. Atopic dermatitis can have a negative impact on children’s development and schooling.
The condition is not common everywhere. According to the 2017 Global Burden of Disease (GBD) data, Sweden, the U.K., Iceland, Finland, and Denmark had the five highest scores, while Uzbekistan, Armenia, Tajikistan, China, and Kazakhstan had the five lowest scores. Hence, rise in incidence of atopic dermatitis across the globe and increase in awareness about the disease are projected to drive the atopic dermatitis drugs market demand during the forecast period.
In terms of product type, the biologics segment accounted for the largest global atopic dermatitis drugs market share in 2022. This is ascribed to increase in the number of biologic drug approvals in the past few years. Biologics are considered to represent a breakthrough in the management of atopic dermatitis because of the improvement seen in clinical outcomes and the quality of life of patients.
Biologics work differently than other atopic dermatitis treatments. Instead of treating general inflammation all over the body, they target specific molecules and do not go through the whole system. Hence, they tend to cause fewer side effects than other treatments. This is propelling the biologics segment.
Recent approvals and launches, such as Abbvie’s Rinvoq, Pfizer’s Cibinqo, and Incyte’s Opzelura, indicate that demand for biologic drugs in the treatment of atopic dermatitis is rising across the globe, especially in the U.S. and Europe, where large populations are affected.
Based on distribution channel, the hospital pharmacies segment dominated the global atopic dermatitis drugs market in 2022. Distribution channels for atopic dermatitis, including biologics, can vary depending on the region and healthcare system.
Atopic dermatitis can be treated with a range of medications, including topical corticosteroids, immunomodulators, emollients, and biologics. These medications are largely available at hospital pharmacies. Hence, the hospital pharmacies segment dominated the global atopic dermatitis drugs market in 2022.
Atopic dermatitis is relatively common in North America, with a significant number of individuals affected by the condition. This high prevalence drives demand for effective treatments and contributes to the expansion of the market.
As per atopic dermatitis drugs market research, the U.S. is likely to dominate the industry during the forecast period owing to its large patient population and usage of biologic drugs in the affected population of patients with atopic dermatitis.
According to the National Eczema Association, around 9.6 million U.S. children under the age of 18 have atopic dermatitis, and one-third have moderate-to-severe disease. An estimated 16.5 million U.S. adults (7.3%) have atopic dermatitis, with nearly 40% affected by moderate-to-severe disease.
North America boasts a well-developed healthcare infrastructure, including hospitals, specialized dermatology clinics, research institutions, and pharmaceutical companies. This infrastructure supports the diagnosis, treatment, and development of new therapies for atopic dermatitis.
The global atopic dermatitis drugs market is consolidated, with the presence of small number of leading players. Expansion of product portfolio and mergers & acquisitions are the key strategies implemented by the leading players in the global industry.
Sanofi, Pfizer, AbbVie, Leo Pharma, Eli Lilly, Teva Pharmaceutical Industries Ltd., and Novartis AG are the prominent players in the global atopic dermatitis drugs market.
Each of these players has been profiled in the atopic dermatitis drugs market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2022 | US$ 11.5 Bn |
| Market Forecast Value in 2031 | More than US$ 28.2 Bn |
| Growth Rate (CAGR) | 8.9% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2021 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Furthermore, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Market Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 11.5 Bn in 2021
It is projected to reach more than US$ 28.2 Bn by 2031
The CAGR is anticipated to be 8.9% from 2023 to 2031
Rise in prevalence of atopic dermatitis and increase in demand for biologics and new product approvals
North America is likely to account for significant share during the forecast period
Sanofi, Pfizer, AbbVie, Leo Pharma, Eli Lilly, Teva Pharmaceutical Industries Ltd, and Novartis AG
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary : Global Atopic Dermatitis Drugs Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Atopic Dermatitis Drugs Market Analysis and Forecast, 2017-2031
5. Key Insights
5.1. Pipeline Analysis
5.2. Disease Prevalence Assessment, by Eczema
5.3. Key Industry Events (Mergers & Acquisitions, Partnerships, Product Launches, etc.)
5.4. COVID-19 Impact Analysis
6. Global Atopic Dermatitis Drugs Market Analysis and Forecast, by Product Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Product Type, 2017-2031
6.3.1. Biologics
6.3.2. Others
6.4. Market Attractiveness, by Product Type
7. Global Atopic Dermatitis Drugs Market Analysis and Forecast, by Distribution Channel
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Distribution Channel, 2017-2031
7.3.1. Hospital Pharmacies
7.3.2. Retail Pharmacies
7.3.3. Online Pharmacies
7.4. Market Attractiveness, by Distribution Channel
8. Global Atopic Dermatitis Drugs Market Analysis and Forecast, by Region
8.1. Key Findings
8.2. Market Value Forecast, by Region
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Latin America
8.2.5. Middle East & Africa
8.3. Market Attractiveness, by Region
9. North America Atopic Dermatitis Drugs Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value Forecast, by Product Type, 2017-2031
9.2.1. Biologics
9.2.2. Others
9.3. Market Value Forecast, by Distribution Channel, 2017-2031
9.3.1. Hospital Pharmacies
9.3.2. Retail Pharmacies
9.3.3. Online Pharmacies
9.4. Market Value Forecast, by Country, 2017-2031
9.4.1. U.S.
9.4.2. Canada
9.5. Market Attractiveness Analysis
9.5.1. By Product Type
9.5.2. By Distribution Channel
9.5.3. By Country
10. Europe Atopic Dermatitis Drugs Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Product Type, 2017-2031
10.2.1. Biologics
10.2.2. Others
10.3. Market Value Forecast, by Distribution Channel, 2017-2031
10.3.1. Hospital Pharmacies
10.3.2. Retail Pharmacies
10.3.3. Online Pharmacies
10.4. Market Value Forecast, by Country/Sub-region, 2017-2031
10.4.1. Germany
10.4.2. U.K.
10.4.3. France
10.4.4. Italy
10.4.5. Spain
10.4.6. Rest of Europe
10.5. Market Attractiveness Analysis
10.5.1. By Product Type
10.5.2. By Distribution Channel
10.5.3. By Country/Sub-region
11. Asia Pacific Atopic Dermatitis Drugs Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Product Type, 2017-2031
11.2.1. Biologics
11.2.2. Others
11.3. Market Value Forecast, by Distribution Channel, 2017-2031
11.3.1. Hospital Pharmacies
11.3.2. Retail Pharmacies
11.3.3. Online Pharmacies
11.4. Market Value Forecast, by Country/Sub-region, 2017-2031
11.4.1. China
11.4.2. Japan
11.4.3. India
11.4.4. Australia & New Zealand
11.4.5. Rest of Asia Pacific
11.5. Market Attractiveness Analysis
11.5.1. By Product Type
11.5.2. By Distribution Channel
11.5.3. By Country/Sub-region
12. Latin America Atopic Dermatitis Drugs Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Product Type, 2017-2031
12.2.1. Biologics
12.2.2. Others
12.3. Market Value Forecast, by Distribution Channel, 2017-2031
12.3.1. Hospital Pharmacies
12.3.2. Retail Pharmacies
12.3.3. Online Pharmacies
12.4. Market Value Forecast, by Country/Sub-region, 2017-2031
12.4.1. Brazil
12.4.2. Mexico
12.4.3. Rest of Latin America
12.5. Market Attractiveness Analysis
12.5.1. By Product Type
12.5.2. By Distribution Channel
12.5.3. By Country/Sub-region
13. Middle East & Africa Atopic Dermatitis Drugs Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Product Type, 2017-2031
13.2.1. Biologics
13.2.2. Others
13.3. Market Value Forecast, by Distribution Channel, 2017-2031
13.3.1. Hospital Pharmacies
13.3.2. Retail Pharmacies
13.3.3. Online Pharmacies
13.4. Market Value Forecast, by Country/Sub-region, 2017-2031
13.4.1. GCC Countries
13.4.2. South Africa
13.4.3. Rest of Middle East & Africa
13.5. Market Attractiveness Analysis
13.5.1. By Product Type
13.5.2. By Distribution Channel
13.5.3. By Country/Sub-region
14. Competition Landscape
14.1. Market Player - Competitive Matrix (by tier and size of companies)
14.2. Market Share Analysis, by Company (2022)
14.3. Company Profiles
14.3.1. Sanofi
14.3.1.1. Company Overview
14.3.1.2. Product Portfolio
14.3.1.3. Financial Overview
14.3.1.4. Strategic Overview
14.3.1.5. SWOT Analysis
14.3.2. Pfizer
14.3.2.1. Company Overview
14.3.2.2. Product Portfolio
14.3.2.3. Financial Overview
14.3.2.4. Strategic Overview
14.3.2.5. SWOT Analysis
14.3.3. AbbVie
14.3.3.1. Company Overview
14.3.3.2. Product Portfolio
14.3.3.3. Financial Overview
14.3.3.4. Strategic Overview
14.3.3.5. SWOT Analysis
14.3.4. Leo Pharma
14.3.4.1. Company Overview
14.3.4.2. Product Portfolio
14.3.4.3. Financial Overview
14.3.4.4. Strategic Overview
14.3.4.5. SWOT Analysis
14.3.5. Eli Lilly and Company
14.3.5.1. Company Overview
14.3.5.2. Product Portfolio
14.3.5.3. Financial Overview
14.3.5.4. Strategic Overview
14.3.5.5. SWOT Analysis
14.3.6. Novartis AG
14.3.6.1. Company Overview
14.3.6.2. Product Portfolio
14.3.6.3. Financial Overview
14.3.6.4. Strategic Overview
14.3.6.5. SWOT Analysis
14.3.7. Teva Pharmaceutical Industries Ltd.
14.3.7.1. Company Overview
14.3.7.2. Product Portfolio
14.3.7.3. Financial Overview
14.3.7.4. Strategic Overview
14.3.7.5. SWOT Analysis
List of Tables
Table 01: Global Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Product Type, 2017-2031
Table 02: Global Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 03: Global Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Region, 2017-2031
Table 04: North America Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Country, 2017-2031
Table 05: North America Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Product Type, 2017-2031
Table 06: North America Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 07: Europe Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 08: Europe Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Product Type, 2017-2031
Table 09: Europe Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 10: Asia Pacific Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 11: Asia Pacific Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Product Type, 2017-2031
Table 12: Asia Pacific Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 13: Latin America Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 14: Latin America Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Product Type, 2017-2031
Table 15: Latin America Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 16: Middle East & Africa Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 17: Middle East & Africa Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Product Type, 2017-2031
Table 18: Middle East & Africa Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast, by Distribution Channel, 2017-2031
List of Figures
Figure 01: Global Atopic Dermatitis Drugs Market Size (US$ Mn) and Distribution (%), by Region, 2017 and 2031
Figure 02: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn), by Product Type, 2021
Figure 03: Global Atopic Dermatitis Drugs Market Value Share, by Product Type, 2021
Figure 04: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn), by Distribution Channel, 2021
Figure 05: Global Atopic Dermatitis Drugs Market Value Share, by Distribution Channel, 2021
Figure 06: Global Atopic Dermatitis Drugs Market Value Share, by Region, 2021
Figure 07: Global Atopic Dermatitis Drugs Market Value (US$ Mn) Forecast, 2017-2031
Figure 08: Global Atopic Dermatitis Drugs Market Value Share Analysis, by Product Type, 2017 and 2031
Figure 09: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Biologics, 2017-2031
Figure 10: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2017-2031
Figure 11: Global Atopic Dermatitis Drugs Market Attractiveness Analysis, by Product Type, 2022-2031
Figure 12: Global Atopic Dermatitis Drugs Market Value Share Analysis, by Distribution Channel, 2017 and 2031
Figure 13: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Hospital Pharmacies, 2017-2031
Figure 14: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Retail Pharmacies, 2017-2031
Figure 15: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Online Pharmacies, 2017-2031
Figure 16: Global Atopic Dermatitis Drugs Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2017-2031
Figure 17: Global Atopic Dermatitis Drugs Market Attractiveness Analysis, by Distribution Channel, 2022-2031
Figure 18: Global Atopic Dermatitis Drugs Market Value Share Analysis, by Region, 2017 and 2031
Figure 19: Global Atopic Dermatitis Drugs Market Attractiveness Analysis, by Region, 2022-2031
Figure 20: North America Atopic Dermatitis Drugs Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017-2031
Figure 21: North America Atopic Dermatitis Drugs Market Attractiveness Analysis, by Country, 2017-2031
Figure 22: North America Atopic Dermatitis Drugs Market Value Share Analysis, by Country, 2017 and 2031
Figure 23: North America Atopic Dermatitis Drugs Market Value Share Analysis, by Product Type, 2017 and 2031
Figure 24: North America Atopic Dermatitis Drugs Market Value Share Analysis, by Distribution Channel, 2017 and 2031
Figure 25: North America Atopic Dermatitis Drugs Market Attractiveness Analysis, by Product Type, 2022-2031
Figure 26: North America Atopic Dermatitis Drugs Market Attractiveness Analysis, by Distribution Channel, 2022-2031
Figure 27: Europe Atopic Dermatitis Drugs Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017-2031
Figure 28: Europe Atopic Dermatitis Drugs Market Attractiveness Analysis, by Country/Sub-region, 2017-2031
Figure 29: Europe Atopic Dermatitis Drugs Market Value Share Analysis, by Country/Sub-region, 2017 and 2031
Figure 30: Europe Atopic Dermatitis Drugs Market Value Share Analysis, by Product Type, 2017 and 2031
Figure 31: Europe Atopic Dermatitis Drugs Market Value Share Analysis, by Distribution Channel, 2017 and 2031
Figure 32: Europe Atopic Dermatitis Drugs Market Attractiveness Analysis, by Product Type, 2022-2031
Figure 33: Europe Atopic Dermatitis Drugs Market Attractiveness Analysis, by Distribution Channel, 2022-2031
Figure 34: Asia Pacific Atopic Dermatitis Drugs Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017-2031
Figure 35: Asia Pacific Atopic Dermatitis Drugs Market Attractiveness Analysis, by Country/Sub-region, 2017-2031
Figure 36: Asia Pacific Atopic Dermatitis Drugs Market Value Share Analysis, by Country/Sub-region, 2017 and 2031
Figure 37: Asia Pacific Atopic Dermatitis Drugs Market Value Share Analysis, by Product Type, 2017 and 2031
Figure 38: Asia Pacific Atopic Dermatitis Drugs Market Value Share Analysis, by Distribution Channel, 2017 and 2031
Figure 39: Asia Pacific Atopic Dermatitis Drugs Market Attractiveness Analysis, by Product Type, 2022-2031
Figure 40: Asia Pacific Atopic Dermatitis Drugs Market Attractiveness Analysis, by Distribution Channel, 2022-2031
Figure 41: Latin America Atopic Dermatitis Drugs Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017-2031
Figure 42: Latin America Atopic Dermatitis Drugs Market Attractiveness Analysis, by Country/Sub-region, 2017-2031
Figure 43: Latin America Atopic Dermatitis Drugs Market Value Share Analysis, by Country/Sub-region, 2017 and 2031
Figure 44: Latin America Atopic Dermatitis Drugs Market Value Share Analysis, by Product Type, 2017 and 2031
Figure 45: Latin America Atopic Dermatitis Drugs Market Value Share Analysis, by Distribution Channel, 2017 and 2031
Figure 46: Latin America Atopic Dermatitis Drugs Market Attractiveness Analysis, by Product Type, 2022-2031
Figure 47: Latin America Atopic Dermatitis Drugs Market Attractiveness Analysis, by Distribution Channel, 2022-2031
Figure 48: Middle East & Africa Atopic Dermatitis Drugs Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2017-2031
Figure 49: Middle East & Africa Atopic Dermatitis Drugs Market Attractiveness Analysis, by Country/Sub-region, 2017-2031
Figure 50: Middle East & Africa Atopic Dermatitis Drugs Market Value Share Analysis, by Country/Sub-region, 2017 and 2031
Figure 51: Middle East & Africa Atopic Dermatitis Drugs Market Value Share Analysis, by Product Type, 2017 and 2031
Figure 52: Middle East & Africa Atopic Dermatitis Drugs Market Value Share Analysis, by Distribution Channel, 2017 and 2031
Figure 53: Middle East & Africa Atopic Dermatitis Drugs Market Attractiveness Analysis, by Product Type, 2022-2031
Figure 54: Middle East & Africa Atopic Dermatitis Drugs Market Attractiveness Analysis, by Distribution Channel, 2022-2031