Reports
Reports
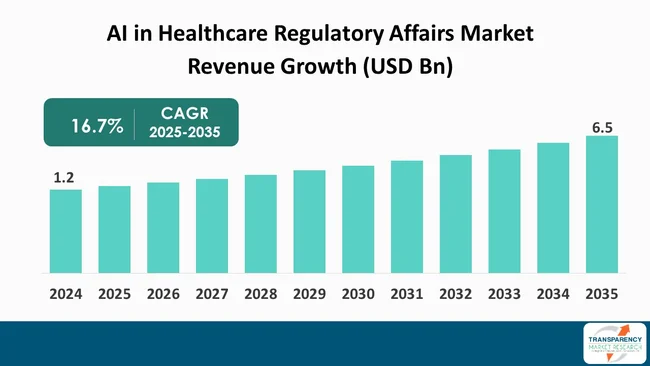
The global AI in healthcare regulatory affairs market size was valued at US$ 1.2 Bn in 2024 and is projected to reach US$ 6.5 Bn by 2035, expanding at a CAGR of 16.7% from 2025 to 2035. The market growth is driven by the increasing complexity of global regulations, need for greater efficiency and speed in drug development and submissions, and advancements in AI technologies for risk prediction.
One of the major factors responsible for adopting AI in healthcare regulatory affairs is an evident need for reduction in time-to-market and costs associated with it. Automating document management, CI/CT submission, and dossier assembly makes the process quicker while minimizing human errors. The use of AI-powered workflow orchestration by regulatory personnel helps to get approvals faster, enhance compliance monitoring, and optimize resource allocation.
Increase in the use of real-world and clinical data as well as the need for continuous pharmacovigilance have led to the adoption of AI in healthcare regulatory affairs market growth. AI enables scalable detection of safety signals, tracking of adverse events, and benefit-risk analysis across different data sets. Improved harmonization of data and automated literature surveillance enable regulatory personnel to focus on strategic decision-making and effective policy interpretation.
Recent trends comprise the use of natural language processing for regulatory intelligence, explainable AI deployment based on the need for auditability, and the widespread use of cloud-native regulatory platforms for collaborative submissions. Therefore, regulation is being met with reduced friction due to higher interoperability standards, API-driven information exchange, and embedding compliance-by-design in the product development lifecycle.
Platformization and modular offerings combining regulatory intelligence, submission automation, and safety analytics are the major factors that define the competitive landscape. Key initiatives include investments in explainability and traceability and expanding managed services to support regional regulatory filings. A heightened strategic focus on customer experience and configurable workflows supports different regulatory frameworks and compliance models.
AI in healthcare regulatory affairs involve the use of machine learning, natural language processing (NLP), and automation software in streamlining and maximizing regulatory processes across healthcare. The solutions facilitate the management of an extensive volume of regulatory information, ensure adherence to worldwide regulations, and contribute to the accuracy and value of submissions.
Artificial intelligence-based systems have the capability to comprehend unstructured regulatory documents, extract essential facts, and identify areas where compliance is lacking.
AI is extensively used in various stages of drug development and medical device approval to streamline tasks related to dossier compilation, clinical trial monitoring, safety data assessment, and post-market surveillance. Automation of these processes can help companies to speed up their approvals and keep up with the requirements of agencies such as FDA and EMA, thus, saving money, and lowering the risk of their operations.
In a nutshell, AI-powered solutions in healthcare regulatory affairs act as a major shift from the traditional compliance framework by making it possible to have predictive insights, regulatory intelligence, and digital automation. These technologies give the healthcare industry the capability to increase productivity, deepen their decision-making process, and attain regulatory outcomes that are quicker and more dependable in the different markets worldwide.
| Attribute | Detail |
|---|---|
| AI in Healthcare Regulatory Affairs Market Drivers |
|
The rising complexity of global regulations is a significant driver to the AI in healthcare regulatory affairs market. As regulatory agencies across geographies continue to change and augment their frameworks, companies are increasingly struggling to maintain compliance for several jurisdictions. This has driven the demand for smart systems that track, analyze, and change to evolving regulatory landscapes effectively.
AI-based technologies provide strong solutions to course through this complexity by tracking regulatory changes and analyzing large amounts of guidance documents, policies, and compliance notifications themselves.
Moreover, AI-powered systems enable cross-border regulatory submissions by standardizing different documentation requirements. Such technologies make it easy for the different regional data formats to be combined, carry out translation jobs automatically, and set compliance practices in line with international standards, thus reducing redundancy and manual workload for regulatory professionals.
In conclusion, the intricacy of worldwide regulations propels healthcare companies to adopt AI-based regulatory solutions to improve their operational efficiency, accuracy, and speed. By simplifying regulatory intelligence gathering and compliance management, AI empowers organizations to handle multi-market operations effectively and minimize the risks of non-compliance in a drastically regulated environment.
Emerging innovations in AI technologies are a considerable driver to the AI in healthcare regulatory affairs market. NLP (Natural Language Processing), machine learning, and predictive analytics are revolutionizing regulatory teams engage with complex data and compliance documents. AI technologies facilitate the attainment of higher degrees of automation, precision, and magnitude in responding to global regulatory demands.
AI-based applications can interpret unstructured regulatory documents, analyze safety reports, and identify possible future compliance issues with flawless accuracy. NLP integration allows systems to extract pertinent insights from scientific publications, guidelines, and submission data, thus, decreasing human workload and decision time.
The development of predictive AI models for organizations is also instrumental in predicting future regulatory risks and anticipating timelines for approval. It enables companies to address compliance issues, optimize an effective submission strategy, thereby ensuring earlier access to the market for new drugs and medical devices.
Essentially, one of the main reasons for the growing popularity of AI-powered compliance tools is the continuous AI technological innovations over time. Such intricate systems do not only upgrade the operational efficiency and openness, but they also help in data harmonization, traceability and audit readiness. As a result, the arrival of sophisticated AI tools is leading the change in healthcare regulatory affairs.
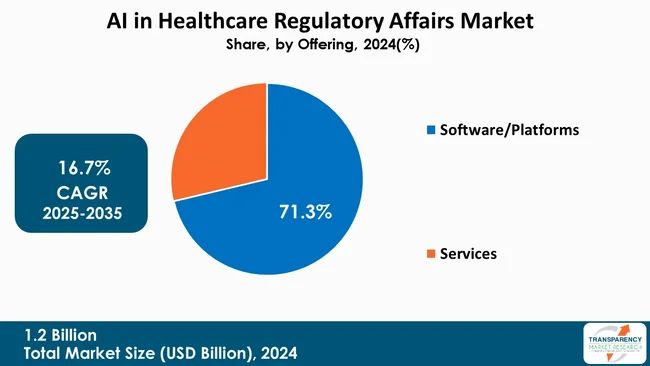
The software/platform segment is leading the AI in healthcare regulatory affairs market due to its ability to centralize and automate intricate regulatory processes. The platforms efficiently manage documents, track submission, and report compliance, which results in the reduction of manual errors and improvement of operational efficiency. Because of their scalability and integration features, they are suitable for global regulatory management.
Moreover, cloud-based platforms ensure the availability of data in real-time, advanced analytics, and facilitation of the collaboration between different teams. The increased use of AI-powered tools for regulatory intelligence, data harmonization, and workflow automation is the main reason behind the segment’s dominance, thus allowing organizations to enjoy benefits of faster approvals, enhanced compliance, and better decision-making across various markets.
| Attribute | Detail |
|---|---|
| Leading Region |
|
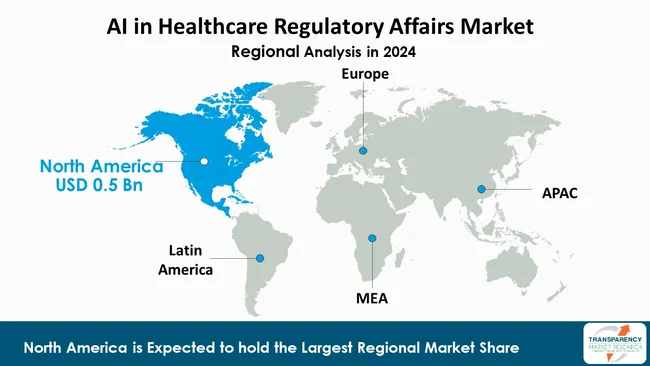
As per the latest AI in healthcare regulatory affairs market analysis, North America led in 2024, with an estimated market share of 41.6%. This can be attributed to the region’s strong AI technology infrastructure, robust regulatory ecosystem, and high healthcare R&D spending. Supportive initiatives from the U.S. FDA, coupled with early adoption by leading biotechnology, pharmaceutical, and medtech companies have accelerated integration of AI into submission management, compliance, and safety monitoring workflows.
Moreover, government funding for AI-driven innovation and collaborations between regulators, industry, and academia continue to foster rapid technology validation and commercialization across regulatory affairs functions.
The companies operating in the AI in healthcare regulatory affairs environment emphasize on strategic collaboration, platform integration, and AI-powered automation to improve compliance efficiency. These firms invest on predictive analytics, cloud-based solutions, and regulatory intelligence tools, and at the same time, they are expanding their service portfolios and geographical reach to be able to provide the full range of regulatory support.
Clarivate, IQVIA Inc., Wipro, Freyr, Innoplexus, Zenovel, Indegene, RegDesk, Inc., CELEGENCE, Rimsys, DDi. DXC Technology Company, Ketryx Corporation are some of the leading players operating in the global AI in healthcare regulatory affairs market.
Each of these players has been profiled in the AI in healthcare regulatory affairs market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2024 | US$ 1.2 Bn |
| Forecast Value in 2035 | US$ 6.5 Bn |
| CAGR | 16.7% |
| Forecast Period | 2025-2035 |
| Historical Data Available for | 2020-2023 |
| Quantitative Units | US$ Bn |
| AI in Healthcare Regulatory Affairs Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation | Offering
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
The global AI in healthcare regulatory affairs market was valued at US$ 1.2 Bn in 2024
The global AI in healthcare regulatory affairs industry is projected to reach more than US$ 6.5 Bn by the end of 2035
The increasing complexity of global regulations, need for greater efficiency and speed in drug development and submissions, rising cost pressures on healthcare providers, and advancements in AI technologies for risk prediction, compliance management are some of the factors driving the expansion of AI in healthcare regulatory affairs market.
The CAGR is anticipated to be 16.7% from 2025 to 2035
Clarivate, IQVIA Inc., Wipro, Freyr, Innoplexus, Zenovel, Indegene, RegDesk, Inc., CELEGENCE, Rimsys, RegDesk, Inc., Innoplexus, DDi., DXC Technology Company, and Ketryx Corporation
Table 01: Global AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Offering, 2020 to 2035
Table 02: Global AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Deployment Mode, 2020 to 2035
Table 03: Global AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Application, 2020 to 2035
Table 04: Global AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 05: Global AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Region, 2020 to 2035
Table 06: North America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, by Country, 2020-2035
Table 07: North America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Offering, 2020 to 2035
Table 08: North America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Deployment Mode, 2020 to 2035
Table 09: North America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Application, 2020 to 2035
Table 10: North America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 11: Europe AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020-2035
Table 12: Europe AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Offering, 2020 to 2035
Table 13: Europe AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Deployment Mode, 2020 to 2035
Table 14: Europe AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Application, 2020 to 2035
Table 15: Europe AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 16: Asia Pacific AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020-2035
Table 17: Asia Pacific AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Offering, 2020 to 2035
Table 18: Asia Pacific AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Deployment Mode, 2020 to 2035
Table 19: Asia Pacific AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Application, 2020 to 2035
Table 20: Asia Pacific AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 21: Latin America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020-2035
Table 22: Latin America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Offering, 2020 to 2035
Table 23: Latin America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Deployment Mode, 2020 to 2035
Table 24: Latin America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Application, 2020 to 2035
Table 25: Latin America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 26: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020-2035
Table 27: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Offering, 2020 to 2035
Table 28: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Deployment Mode, 2020 to 2035
Table 29: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By Application, 2020 to 2035
Table 30: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Figure 01: Global AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Offering, 2024 and 2035
Figure 02: Global AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Offering, 2025 to 2035
Figure 03: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Software/Platforms, 2020 to 2035
Figure 04: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Services, 2020 to 2035
Figure 05: Global AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Deployment Mode, 2024 and 2035
Figure 06: Global AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Deployment Mode, 2025 to 2035
Figure 07: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Cloud-based, 2020 to 2035
Figure 08: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by On-Premises, 2020 to 2035
Figure 09: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Hybrid, 2020 to 2035
Figure 10: Global AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Application, 2024 and 2035
Figure 11: Global AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 12: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Regulatory Strategy & Insights, 2020 to 2035
Figure 13: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Product Registration & Approvals, 2020 to 2035
Figure 14: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Dossier Authoring, 2020 to 2035
Figure 15: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Pharmacovigilance & Safety Reporting, 2020 to 2035
Figure 16: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Data Migration & Integration, 2020 to 2035
Figure 17: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Regulatory Submissions & Safety Reporting, 2020 to 2035
Figure 18: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Others, 2020 to 2035
Figure 19: Global AI in Healthcare Regulatory Affairs Market Value Share Analysis, By End-user, 2024 and 2035
Figure 20: Global AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 21: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Pharmaceutical and Biotechnology Companies, 2020 to 2035
Figure 22: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Medical Device Manufacturers, 2020 to 2035
Figure 23: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by CRO/CDMO, 2020 to 2035
Figure 24: Global AI in Healthcare Regulatory Affairs Market Revenue (US$ Bn), by Others, 2020 to 2035
Figure 25: Global AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Region, 2024 and 2035
Figure 26: Global AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Region, 2025 to 2035
Figure 27: North America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 28: North America AI in Healthcare Regulatory Affairs Market Value Share Analysis, by Country, 2024 and 2035
Figure 29: North America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, by Country, 2025 to 2035
Figure 30: North America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Offering, 2024 and 2035
Figure 31: North America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Offering, 2025 to 2035
Figure 32: North America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Deployment Mode, 2024 and 2035
Figure 33: North America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Deployment Mode, 2025 to 2035
Figure 34: North America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Application, 2024 and 2035
Figure 35: North America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 36: North America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By End-user, 2024 and 2035
Figure 37: North America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 38: Europe AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 39: Europe AI in Healthcare Regulatory Affairs Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 40: Europe AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 41: Europe AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Offering, 2024 and 2035
Figure 42: Europe AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Offering, 2025 to 2035
Figure 43: Europe AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Deployment Mode, 2024 and 2035
Figure 44: Europe AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Deployment Mode, 2025 to 2035
Figure 45: Europe AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Application, 2024 and 2035
Figure 46: Europe AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 47: Europe AI in Healthcare Regulatory Affairs Market Value Share Analysis, By End-user, 2024 and 2035
Figure 48: Europe AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 49: Asia Pacific AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 50: Asia Pacific AI in Healthcare Regulatory Affairs Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 51: Asia Pacific AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 52: Asia Pacific AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Offering, 2024 and 2035
Figure 53: Asia Pacific AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Offering, 2025 to 2035
Figure 54: Asia Pacific AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Deployment Mode, 2024 and 2035
Figure 55: Asia Pacific AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Deployment Mode, 2025 to 2035
Figure 56: Asia Pacific AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Application, 2024 and 2035
Figure 57: Asia Pacific AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 58: Asia Pacific AI in Healthcare Regulatory Affairs Market Value Share Analysis, By End-user, 2024 and 2035
Figure 59: Asia Pacific AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 60: Latin America AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 61: Latin America AI in Healthcare Regulatory Affairs Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 62: Latin America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 63: Latin America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Offering, 2024 and 2035
Figure 64: Latin America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Offering, 2025 to 2035
Figure 65: Latin America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Deployment Mode, 2024 and 2035
Figure 66: Latin America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Deployment Mode, 2025 to 2035
Figure 67: Latin America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Application, 2024 and 2035
Figure 68: Latin America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 69: Latin America AI in Healthcare Regulatory Affairs Market Value Share Analysis, By End-user, 2024 and 2035
Figure 70: Latin America AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 71: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 72: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 73: Middle East & Africa AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 74: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Offering, 2024 and 2035
Figure 75: Middle East & Africa AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Offering, 2025 to 2035
Figure 76: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Deployment Mode, 2024 and 2035
Figure 77: Middle East & Africa AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Deployment Mode, 2025 to 2035
Figure 78: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value Share Analysis, By Application, 2024 and 2035
Figure 79: Middle East & Africa AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 80: Middle East & Africa AI in Healthcare Regulatory Affairs Market Value Share Analysis, By End-user, 2024 and 2035
Figure 81: Middle East & Africa AI in Healthcare Regulatory Affairs Market Attractiveness Analysis, By End-user, 2025 to 2035