Reports
Reports
Analyst Viewpoint
Growth in incidence of scleroderma and R&D of new therapeutics are expected to propel the scleroderma diagnostics and therapeutics market size during the forecast period. Various healthcare organizations are boosting awareness regarding the need for early diagnosis and treatment of scleroderma.
Preventive scleroderma management is gaining traction among healthcare professionals. Vendors in the market are conducting various studies for the treatment of Raynaud’s disease in scleroderma patients. They are seeking approval and launch of targeted therapies for scleroderma-associated complications to expand their product portfolio and increase their scleroderma diagnostics and therapeutics market share.
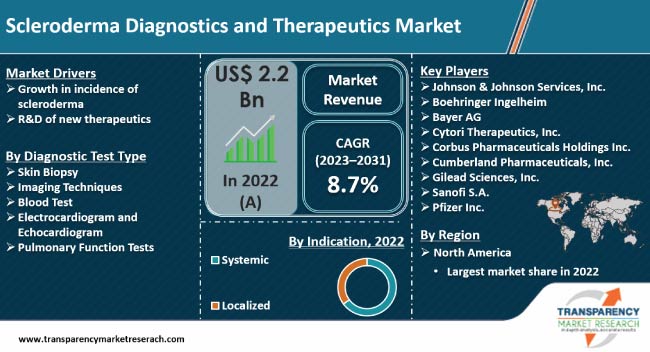
Scleroderma is an autoimmune disease characterized by chronic hardening and tightening of the skin and connective tissues There are two main types of scleroderma: localized scleroderma and systemic sclerosis (systemic scleroderma). This classification is based on the severity of skin thickening, which adversely affects kidneys, lungs, gastrointestinal systems, and the heart.
Systemic sclerosis diagnostics can be challenging because its symptoms are diverse and can mimic other conditions. Vendors in the global scleroderma diagnostics and therapeutics industry are investing in R&D activities in scleroderma and autoimmune diseases and several first-in-class curatives are under clinical development. Approval and launch of novel therapeutics is expected to spur the scleroderma diagnostics and therapeutics market growth in the near future.
Scleroderma is a complex autoimmune disease. Various studies are focusing on identifying the specific proteins or molecular pathways involved in the pathogenesis of the disease. ‘LMCD1’ protein could be a potential therapeutic target for patients suffering from systemic sclerosis. Those infected with interstitial lung disease (ILD) have a higher presence of LMCD1 protein as compared to healthy people. Thus, various studies are focused on LMCD1 as a potential therapeutic target for patients suffering from scleroderma. Rise in investment in such studies is projected to augment the scleroderma diagnostics and therapeutics market value in the next few years.
Some studies have reported that an exclusive macrophage – an immune cell capable of removing dead cells or bacteria, plays an important role in scarring in lungs and skin along with chronic inflammation. These studies are looking forward to targeting this macrophage for the development of effective scleroderma therapeutics.
A study published in the Journal of Scleroderma and Related Disorders (JSRD) in February 2022 reported that immunosuppressive drugs (methotrexate, in particular) help reduce scleroderma symptoms to a greater extent. In October 2022, clinicians from the Michigan Medicine and the University of Pittsburgh received approval from the U.S. FDA for tofacitinib for the treatment of scleroderma. Thus, approval and launch of new therapeutics is fueling the scleroderma diagnostics and therapeutics market progress during the forecast period.
Scleroderma is common in women aged between 30 and 50 who undergo pregnancy, lactating, and menopause. Raynaud’s phenomenon is one of the earliest manifestations of this ailment. It is characterized by decreased blood flow to the fingers (vasospasms) and is induced by stress or cold temperature. Finger ulcers, joint and muscle pain, dilated blood vessels, and skin tightening could develop if the condition is not treated on time. According to the National Organization for Rare Disorders, systemic scleroderma affects 38 to 341 individuals per million throughout the world and develops in 8 to 56 individuals per million each year.
Systemic sclerosis is linked with noticeable morbidity inclusive of disability, pain, depression, and reduced quality of life. The subsequent effects include a reduction in physical activity or physical capacity to perform daily chores. This ailment may result in the weakening of muscles along with impairment in oxygen consumption and transport. Therefore, healthcare personnel are recommending preventive healthcare. It implies mechanisms such as modulation of redox homeostasis and/or stress-response proteins that enhance functional and muscular performance and endocrine-metabolic, mental, and cardiovascular health.
According to the latest scleroderma diagnostics and therapeutics market forecast, North America is expected to hold largest share from 2023 to 2031. Presence of a well-established healthcare sector and rise in R&D of new therapeutics are fueling market dynamics of the region. Several non-profit organizations are raising awareness regarding scleroderma. The National Scleroderma Foundation focuses on medical research, promotes awareness about scleroderma, and renders support and education to those suffering from the disease.
Surge in incidence of scleroderma is propelling the scleroderma diagnostics and therapeutics market statistics in Europe and Asia Pacific. According to the National Center for Biotechnology Information, the reported prevalence of systemic sclerosis in Europe was 13.5-44.3 per 100,000 individuals. suffer from. The reported prevalence of scleroderma was 24.4 per 100,000 individuals in Thailand.
Most scleroderma diagnostics and therapeutics vendors are conducting phased trials regarding scleroderma treatment. Innovative approaches in scleroderma clinical trials include targeted therapies, stem cell therapy, and precision medicine. Johnson & Johnson Services, Inc., Boehringer Ingelheim, Bayer AG, Cytori Therapeutics, Inc., Corbus Pharmaceuticals Holdings Inc., Cumberland Pharmaceuticals, Inc., Gilead Sciences, Inc., Sanofi S.A., Pfizer Inc., F. Hoffmann-La Roche AG, and Merck & Co., Inc. are key players operating in this market.
Each of these players has been profiled in the scleroderma diagnostics and therapeutics market report based on parameters such as company overview, business strategies, financial overview, product portfolio, and business segments.
| Attribute | Detail |
|---|---|
| Market Value in 2022 (Base Year) | US$ 2.2 Bn |
| Market Forecast Value in 2031 | US$ 4.6 Bn |
| Growth Rate (CAGR) | 8.7% |
| Forecast Period | 2023-2031 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | Qualitative analysis includes drivers, restraints, opportunities, key trends, key market indicators, Porter’s Five Forces analysis, value chain analysis, and SWOT analysis. Furthermore, at the regional level, the qualitative analysis includes key trends, price trends, and key supplier analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Regions Covered |
|
| Countries Covered |
|
| Market Segmentation |
|
| Companies Profiled |
|
| Scope for Customization | Available upon Request |
| Pricing | Available upon Request |
It was valued at US$ 2.2 Bn in 2022
It is projected to grow at a CAGR of 8.7% from 2023 to 2031
Growth in incidence of scleroderma and R&D of new therapeutics
North America is estimated to dominate during the forecast period
Johnson & Johnson Services, Inc., Boehringer Ingelheim, Bayer AG, Cytori Therapeutics, Inc., Corbus Pharmaceuticals Holdings Inc., Cumberland Pharmaceuticals, Inc., Gilead Sciences, Inc., Sanofi S.A., Pfizer Inc., F. Hoffmann-La Roche AG, and Merck & Co., Inc.
1. Executive Summary
1.1. Global Market Outlook
1.2. Demand Side Trends
1.3. Key Facts and Figures
1.4. Trends Impacting Market
1.5. TMR’s Growth Opportunity Wheel
2. Market Overview
2.1. Market Segmentation
2.2. Market Trends
2.3. Market Dynamics
2.3.1. Drivers
2.3.2. Restraints
2.3.3. Opportunities
2.4. Porter’s Five Forces Analysis
2.5. Regulatory Analysis
2.6. Value Chain Analysis
2.6.1. List of Raw Material Suppliers
2.6.2. List of Key Manufacturers
2.6.3. List of Suppliers/ Distributors
2.6.4. List of Potential Customers
2.7. Product Specification Analysis
2.8. Overview of Manufacturing Process
2.9. Cost Structure Analysis
3. COVID-19 Impact Analysis
4. Price Trend Analysis
5. Global Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, by Drug Class, 2023–2031
5.1. Introduction and Definitions
5.2. Global Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
5.2.1. Corticosteroids
5.2.2. Immunosuppressive Agents
5.2.3. Endothelin Receptor Agonists
5.2.4. Calcium Channel Blockers
5.2.5. PD5-Inhibitors
5.2.6. Chelating Agents
5.2.7. Prostacyclin Analogues
5.2.8. H2 Blockers
5.2.9. Proton Pump Inhibitors
5.2.10. ACE Inhibitors
5.3. Global Scleroderma Diagnostics and Therapeutics Market Attractiveness, by Drug Class
6. Global Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, by Indication, 2023–2031
6.1. Introduction and Definitions
6.2. Global Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
6.2.1. Systemic
6.2.2. Localized
6.3. Global Scleroderma Diagnostics and Therapeutics Market Attractiveness, by Indication
7. Global Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, by Diagnostic Test Type, 2023–2031
7.1. Introduction and Definitions
7.2. Global Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
7.2.1. Skin Biopsy
7.2.2. Imaging Techniques
7.2.3. Blood Test
7.2.4. Electrocardiogram and Echocardiogram
7.2.5. Pulmonary Function Tests
7.3. Global Scleroderma Diagnostics and Therapeutics Market Attractiveness, by Diagnostic Test Type
8. Global Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, by Region, 2023–2031
8.1. Key Findings
8.2. Global Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Region, 2023–2031
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Middle East & Africa
8.2.5. Latin America
8.3. Global Scleroderma Diagnostics and Therapeutics Market Attractiveness, by Region
9. North America Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, 2023–2031
9.1. Key Findings
9.2. North America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
9.3. North America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
9.4. North America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
9.5. North America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Country, 2023–2031
9.5.1. U.S. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
9.5.2. U.S. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
9.5.3. U.S. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
9.5.4. Canada Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
9.5.5. Canada Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
9.5.6. Canada Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
9.6. North America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis
10. Europe Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, 2023–2031
10.1. Key Findings
10.2. Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.3. Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.4. Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.5. Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Country and Sub-region, 2023-2031
10.5.1. Germany Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.5.2. Germany Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.5.3. Germany Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.5.4. France Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.5.5. France Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.5.6. France Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.5.7. U.K. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.5.8. U.K. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.5.9. U.K. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.5.10. Italy Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.5.11. Italy. Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.5.12. Italy Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.5.13. Russia & CIS Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.5.14. Russia & CIS Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.5.15. Russia & CIS Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.5.16. Rest of Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
10.5.17. Rest of Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
10.5.18. Rest of Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
10.6. Europe Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis
11. Asia Pacific Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, 2023–2031
11.1. Key Findings
11.2. Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class
11.3. Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
11.4. Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
11.5. Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Country and Sub-region, 2023-2031
11.5.1. China Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
11.5.2. China Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
11.5.3. China Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
11.5.4. Japan Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
11.5.5. Japan Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
11.5.6. Japan Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
11.5.7. India Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
11.5.8. India Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
11.5.9. India Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
11.5.10. ASEAN Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
11.5.11. ASEAN Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
11.5.12. ASEAN Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
11.5.13. Rest of Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
11.5.14. Rest of Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
11.5.15. Rest of Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
11.6. Asia Pacific Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis
12. Latin America Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, 2023–2031
12.1. Key Findings
12.2. Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
12.3. Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
12.4. Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
12.5. Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Country and Sub-region, 2023-2031
12.5.1. Brazil Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
12.5.2. Brazil Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
12.5.3. Brazil Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
12.5.4. Mexico Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
12.5.5. Mexico Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
12.5.6. Mexico Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
12.5.7. Rest of Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
12.5.8. Rest of Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
12.5.9. Rest of Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
12.6. Latin America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis
13. Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Analysis and Forecast, 2023–2031
13.1. Key Findings
13.2. Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
13.3. Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
13.4. Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
13.5. Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Country and Sub-region, 2023-2031
13.5.1. GCC Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
13.5.2. GCC Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
13.5.3. GCC Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
13.5.4. South Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
13.5.5. South Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
13.5.6. South Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
13.5.7. Rest of Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Drug Class, 2023–2031
13.5.8. Rest of Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Indication, 2023–2031
13.5.9. Rest of Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
13.6. Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis
14. Competition Landscape
14.1. Global Scleroderma Diagnostics and Therapeutics Company Market Share Analysis, 2022
14.2. Company Profiles (Details – Overview, Financials, Recent Developments, and Strategy)
14.2.1. Johnson & Johnson Services, Inc.
14.2.1.1. Company Description
14.2.1.2. Business Overview
14.2.1.3. Financial Overview
14.2.1.4. Strategic Overview
14.2.2. Boehringer Ingelheim
14.2.2.1. Company Description
14.2.2.2. Business Overview
14.2.2.3. Financial Overview
14.2.2.4. Strategic Overview
14.2.3. Bayer AG
14.2.3.1. Company Description
14.2.3.2. Business Overview
14.2.3.3. Financial Overview
14.2.3.4. Strategic Overview
14.2.4. Cytori Therapeutics, Inc.
14.2.4.1. Company Description
14.2.4.2. Business Overview
14.2.4.3. Financial Overview
14.2.4.4. Strategic Overview
14.2.5. Corbus Pharmaceuticals Holdings Inc.
14.2.5.1. Company Description
14.2.5.2. Business Overview
14.2.5.3. Financial Overview
14.2.5.4. Strategic Overview
14.2.6. Cumberland Pharmaceuticals, Inc.
14.2.6.1. Company Description
14.2.6.2. Business Overview
14.2.6.3. Financial Overview
14.2.6.4. Strategic Overview
14.2.7. Gilead Sciences, Inc.
14.2.7.1. Company Description
14.2.7.2. Business Overview
14.2.7.3. Financial Overview
14.2.7.4. Strategic Overview
14.2.8. Sanofi S.A.
14.2.8.1. Company Description
14.2.8.2. Business Overview
14.2.8.3. Financial Overview
14.2.8.4. Strategic Overview
14.2.9. Pfizer Inc.
14.2.9.1. Company Description
14.2.9.2. Business Overview
14.2.9.3. Financial Overview
14.2.9.4. Strategic Overview
14.2.10. F. Hoffmann-La Roche AG
14.2.10.1. Company Description
14.2.10.2. Business Overview
14.2.10.3. Financial Overview
14.2.10.4. Strategic Overview
14.2.11. Merck & Co., Inc.
14.2.11.1. Company Description
14.2.11.2. Business Overview
14.2.11.3. Financial Overview
14.2.11.4. Strategic Overview
15. Primary Research: Key Insights
16. Appendix
List of Tables
Table 01: Global Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Drug Class, 2023–2031
Table 02: Global Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Indication, 2023–2031
Table 03: Global Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
Table 04: Global Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Region, 2023–2031
Table 05: North America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Country, 2023–2031
Table 06: North America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Drug Class, 2023–2031
Table 07: North America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Indication, 2023–2031
Table 08: North America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
Table 09: Europe Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Country/Sub-region, 2023–2031
Table 10: Europe Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Drug Class, 2023–2031
Table 11: Europe Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Indication, 2023–2031
Table 12: Europe Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
Table 13: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Country/Sub-region, 2023–2031
Table 14: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Drug Class, 2023–2031
Table 15: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Indication, 2023–2031
Table 16: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
Table 17: Latin America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Country/Sub-region, 2023–2031
Table 18: Latin America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Drug Class, 2023–2031
Table 19: Latin America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Indication, 2023–2031
Table 20: Latin America Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
Table 21: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Country/Sub-region, 2023–2031
Table 22: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Drug Class, 2023–2031
Table 23: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Indication, 2023–2031
Table 24: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast, by Diagnostic Test Type, 2023–2031
List of Figures
Figure 01: Global Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) and Distribution (%), by Region, 2023 and 2031
Figure 02: Global Scleroderma Diagnostics and Therapeutics Market Revenue (US$ Bn), by Drug Class, 2022
Figure 03: Global Scleroderma Diagnostics and Therapeutics Market Value Share, by Drug Class, 2022
Figure 04: Global Scleroderma Diagnostics and Therapeutics Market Revenue (US$ Bn), by Indication, 2022
Figure 05: Global Scleroderma Diagnostics and Therapeutics Market Value Share, by Indication, 2022
Figure 06: Global Scleroderma Diagnostics and Therapeutics Market Revenue (US$ Bn), by Diagnostic Test Type, 2022
Figure 07: Global Scleroderma Diagnostics and Therapeutics Market Value Share, by Diagnostic Test Type, 2022
Figure 08: Global Scleroderma Diagnostics and Therapeutics Market Value Share, by Region, 2022
Figure 09: Global Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast, 2023–2031
Figure 10: Global Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Drug Class, 2023 and 2031
Figure 11: Global Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Drug Class, 2022-2031
Figure 12: Global Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Indication, 2023 and 2031
Figure 13: Global Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Indication, 2022-2031
Figure 14: Global Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Diagnostic Test Type, 2023 and 2031
Figure 15: Global Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Diagnostic Test Type, 2022-2031
Figure 16: Global Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Region, 2023 and 2031
Figure 17: Global Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Region, 2022-2031
Figure 18: North America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast and Y-o-Y Growth (%), 2023–2031
Figure 19: North America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Country, 2023–2031
Figure 20: North America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Country, 2023 and 2031
Figure 21: North America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Drug Class, 2023 and 2031
Figure 22: North America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Indication, 2023 and 2031
Figure 23: North America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Diagnostic Test Type, 2023 and 2031
Figure 24: North America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 25: North America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Indication, 2022–2031
Figure 26:North America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Diagnostic Test Type, 2022–2031
Figure 27: Europe Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast and Y-o-Y Growth (%), 2023–2031
Figure 28: Europe Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 29: Europe Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Country/Sub-region, 2023 and 2031
Figure 30: Europe Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Drug Class, 2023 and 2031
Figure 31: Europe Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Indication, 2023 and 2031
Figure 32: Europe Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Diagnostic Test Type, 2023 and 2031
Figure 33: Europe Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 34: Europe Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Indication, 2022–2031
Figure 35: Europe Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Diagnostic Test Type, 2022–2031
Figure 36: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast and Y-o-Y Growth (%), 2023–2031
Figure 37: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 38: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Country/Sub-region, 2023 and 2031
Figure 39: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Drug Class, 2023 and 2031
Figure 40: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Indication, 2023 and 2031
Figure 41: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Diagnostic Test Type, 2023 and 2031
Figure 42: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 43: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Indication, 2022–2031
Figure 44: Asia Pacific Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Diagnostic Test Type, 2022–2031
Figure 45: Latin America Scleroderma Diagnostics and Therapeutics Market Value (US$ Bn) Forecast and Y-o-Y Growth (%), 2023–2031
Figure 46: Latin America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 47: Latin America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Country/Sub-region, 2023 and 2031
Figure 48: Latin America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Drug Class, 2023 and 2031
Figure 49: Latin America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Indication, 2023 and 2031
Figure 50: Latin America Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Diagnostic Test Type, 2023 and 2031
Figure 51: Latin America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 52: Latin America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Indication, 2022–2031
Figure 53: Latin America Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Diagnostic Test Type, 2022–2031
Figure 54: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Size (US$ Bn) Forecast and Y-o-Y Growth (%), 2023–2031
Figure 55: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 56: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Country/Sub-region, 2023 and 2031
Figure 57: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Drug Class, 2023 and 2031
Figure 58: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Indication, 2023 and 2031
Figure 59: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Value Share Analysis, by Diagnostic Test Type, 2023 and 2031
Figure 60: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 61: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Indication, 2022–2031
Figure 62: Middle East & Africa Scleroderma Diagnostics and Therapeutics Market Attractiveness Analysis, by Diagnostic Test Type, 2022–2031