Reports
Reports
Recombinant factor C (rFC) assay is a synthetic equivalent of Factor C and is the first component of the horseshoe crab clotting cascade, which is activated in response to endotoxins. Moreover, the horseshoe crab blood can be collected only during a short time frame, thus limiting its supply considerably. Hence, healthcare companies in the recombinant factor C assay market are increasing their focus on rFC methods to help alleviate this challenge and improve outcomes.
Novel rFC methods are benefitting healthcare companies, as these companies can limit their reliance on animals or any other source materials. Hence, recombinant factor C assay is acquiring popularity as a sustainable solution for endotoxin testing. Such advancements have led to the growth of the recombinant factor C assay market, where the market is estimated to reach a revenue of ~US$ 28 Mn by the end of 2027. Analysts of Transparency Market Research (TMR) opine that rFC will gain recognition in the endotoxin testing industry for its accuracy and specificity, which is as good as the LAL (Limulus Amebocyte Lysate) assay.
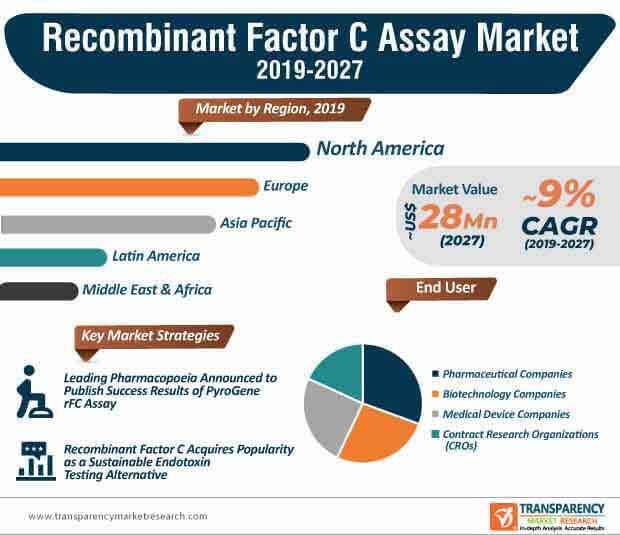
PyroGene™ recombinant factor C assay is gaining market prominence as a single-step enzymatic process as opposed to LAL assay that involves multiple procedural steps. As such, the PyroGene brand is anticipated to dictate the highest revenue as compared to EndoNext in the recombinant factor C assay market where the market is predicted to grow at a favorable CAGR of ~9% during the forecast period. Monitoring samples for contaminants play a pivotal role in pharmaceutical and medical device industries. This explains why the revenue of pharmaceutical companies is estimated to be the highest among all end users in the market landscape.
Traditional LAL testing is largely dependent on the blood of the horseshoe crab namely Limulus polyphemus. However, multiple enzymatic steps associated with LAL assay is majorly dependent on the availability of horseshoe crabs. Hence, companies in the recombinant factor C assay market are increasing their research spending in PyroGene recombinant factor C assay that can be associated with innovative software systems to gain a read-incubate-read sequence within the workflow.
LAL assays are considered as the gold standard for endotoxin testing. However, advantages of recombinant factor C assay are transforming the market landscape, which is dictated by only two major companies. Lonza Group— a leading Swiss multinational biotechnology company is increasing efforts to make its PyroGeneTM recombinant factor C assay a compendial method in Europe by collaborating with the European Pharmacopoeia. On the other hand, Hyglos GmbH— a bioMérieux company, which is a global player in in vitro diagnostics, is increasing efforts to reduce the incidence of false positives from ß-Glucan or interference due to colored or turbid samples. Thus, healthcare companies worldwide should tap opportunities in rFC, since the recombinant factor C assay market is consolidated.
Companies in the recombinant factor C assay market are reducing the pressure on LAL assays for innovations in monitoring samples for contaminants in pharmaceutical products. However, lack of long-term studies that validate rFC for combination testing of unknown samples, inter-laboratory, -operator, and -lot changes poses a barrier for market growth. Hence, pharmaceutical test users are increasing their efficacy in research studies to validate successful outcomes of rFC in specific products.
Effective FDA-approved Drug Builds Credibility of Healthcare Companies
Companies in the recombinant factor C assay market are gaining competitive edge over other players by introducing FDA-approved drugs using rFC. They are increasing efforts to experiment with humanized monoclonal antibodies such as galcanezumab for the prevention of migraine in adults. Novel rFC methods are being highly publicized as non-animal alternatives for the assessment of contaminating endotoxins in drugs. Though LAL is considered as the gold standard for endotoxin testing, official declarations by the FDA are validating the efficiency of PyroGene in the recombinant factor C assay market landscape. Thus, accuracy, sensitivity, and specificity of recombinant factor C assay is being considered at par with LAL methods.
Analysts’ Viewpoint
Gaining FDA approval for drugs is essential in the recombinant factor C assay market. Advantages of rFC are grabbing the attention of commercial innovators in the field of recombinant technology to explore untapped business potentials.
rFC is ensuring the sustainable evolution of horseshoe crab species. However, it is still unsure whether rFC-based tests outshine LAL assays and other robust test systems. Hence, pharmaceutical test users should increase their efficacy in long-term research studies that validate the efficiency of rFC involving unknown samples and inter-laboratory, -operator, and -lot changes. They should increase research in comparative studies of rFC involving kinetic chromogenic LAL assays that help companies to boost their credibility in the market landscape.
Recombinant factor C assay market to reach a revenue of ~US$ 28 Mn by the end of 2027
Recombinant factor C assay market is driven by rise in need for alternative endotoxin detection tests
North America dominated the global recombinant factor C assay market in 2018 and the trend is anticipated to continue during the forecast period
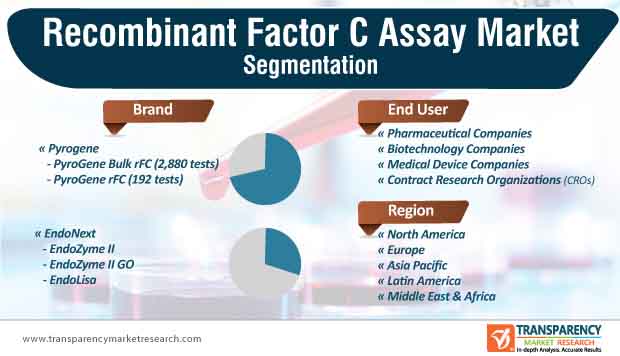
The PyroGene brand segment is likely to account for a major share of the global recombinant factor C assay market during the forecast period
Key players operating in the global recombinant factor C assay market include Lonza Group and Hyglos GmbH – a bioMérieux Company
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Acronyms
3. Research Methodology
4. Executive Summary: Global Recombinant Factor C Assay Market
5. Market Overview
5.1. Definition
5.2. Market Indicators
5.3. Market Dynamics
5.3.1. Drivers
5.3.2. Restraints
5.3.3. Opportunities
5.4. Global Recombinant Factor C Assay Market Revenue (US$ Mn) Forecast 2017–2027
5.5. Global Recombinant Factor C Assay Market Outlook
6. Key Insights
6.1. Value Chain Analysis
6.2. Key Industry Events
6.3. Patent Analysis
6.4. Product Features & USPs
6.5. Overview of Pyrogen Testing Products Market
6.5.1. Pyrogen Testing- Service Providers
6.5.2. Pyrogen Testing Products Marketing, by Country/Region
6.6. Overview of Limulus Amoebocyte Lysate (LAL) Tests
6.6.1. Number of Assays Performed
6.6.2. Cost per Assay and Turn Around Time
6.7. Regulatory Scenario, by Country/Region
6.8. Institutes Involved in Research Studies using Horseshoe Crabs
7. Global Recombinant Factor C Assay Market Analysis and Forecast, by Brand
7.1. Introduction
7.2. Global Recombinant Factor C Assay Market Value Share Analysis, by Brand
7.3. Global Recombinant Factor C Assay Market Value Forecast, by Brand, 2017–2027
7.3.1. PyroGene
7.3.1.1. PyroGene Bulk rFC (2,880 tests)
7.3.1.2. PyroGene rFC (192 tests)
7.3.2. EndoNext
7.3.2.1. Endozyme II
7.3.2.2. Endozyme II GO
7.3.2.3. EndoLisa
7.4. Global Recombinant Factor C Assay Market Attractiveness, by Brand
8. Global Recombinant Factor C Assay Market Analysis and Forecast, by End-user
8.1. Introduction
8.2. Global Recombinant Factor C Assay Market Value Share Analysis, by End-user
8.3. Global Recombinant Factor C Assay Market Value Forecast, by End-user, 2017–2027
8.3.1. Pharmaceutical Companies
8.3.2. Biotechnology Companies
8.3.3. Medical Device Companies
8.3.4. Contract Research Organizations (CROs)
8.4. Global Recombinant Factor C Assay Market Attractiveness, by End-user
9. Global Recombinant Factor C Assay Market Analysis and Forecast, by Region
9.1. Regional Outlook
9.2. Introduction
9.3. Global Recombinant Factor C Assay Market Value Forecast, by Region
9.3.1. North America
9.3.2. Europe
9.3.3. Asia Pacific
9.3.4. Latin America
9.3.5. Middle East & Africa
9.4. Global Recombinant Factor C Assay Market Attractiveness, by Region
10. North America Recombinant Factor C Assay Market Analysis and Forecast
10.1. Key Findings
10.2. North America Recombinant Factor C Assay Market Value Forecast, by Brand, 2017–2027
10.2.1. PyroGene
10.2.1.1. PyroGene Bulk rFC (2,880 tests)
10.2.1.2. PyroGene rFC (192 tests)
10.2.2. EndoNext
10.2.2.1. Endozyme II
10.2.2.2. Endozyme II GO
10.2.2.3. EndoLisa
10.3. North America Recombinant Factor C Assay Market Value Forecast, by End-user, 2017–2027
10.3.1. Pharmaceutical Companies
10.3.2. Biotechnology Companies
10.3.3. Medical Device Companies
10.3.4. Contract Research Organizations (CROs)
10.4. North America Recombinant Factor C Assay Market Value Forecast, by Country, 2017–2027
10.4.1. U.S.
10.4.2. Canada
10.5. North America Recombinant Factor C Assay Market Attractiveness Analysis
10.5.1. By Brand
10.5.2. By End-user
10.5.3. By Country
11. Europe Recombinant Factor C Assay Market Analysis and Forecast
11.1. Key Findings
11.2. Europe Recombinant Factor C Assay Market Value Forecast, by Brand, 2017–2027
11.2.1. PyroGene
11.2.1.1. PyroGene Bulk rFC (2,880 tests)
11.2.1.2. PyroGene rFC (192 tests)
11.2.2. EndoNext
11.2.2.1. Endozyme II
11.2.2.2. Endozyme II GO
11.2.2.3. EndoLisa
11.3. Europe Recombinant Factor C Assay Market Value Forecast, by End-user, 2017–2027
11.3.1. Pharmaceutical Companies
11.3.2. Biotechnology Companies
11.3.3. Medical Device Companies
11.3.4. Contract Research Organizations (CROs)
11.4. Europe Recombinant Factor C Assay Market Value Forecast, by Country/Sub-region, 2017–2027
11.4.1. U.K.
11.4.2. Germany
11.4.3. France
11.4.4. Italy
11.4.5. Spain
11.4.6. Rest of Europe
11.5. Europe Recombinant Factor C Assay Market Attractiveness Analysis
11.5.1. By Brand
11.5.2. By End-user
11.5.3. By Country/Sub-region
12. Asia Pacific Recombinant Factor C Assay Market Analysis and Forecast
12.1. Key Findings
12.2. Asia Pacific Recombinant Factor C Assay Market Value Forecast, by Brand, 2017–2027
12.2.1. PyroGene
12.2.1.1. PyroGene Bulk rFC (2,880 tests)
12.2.1.2. PyroGene rFC (192 tests)
12.2.2. EndoNext
12.2.2.1. Endozyme II
12.2.2.2. Endozyme II GO
12.2.2.3. EndoLisa
12.3. Asia Pacific Recombinant Factor C Assay Market Value Forecast, by End-user, 2017–2027
12.3.1. Pharmaceutical Companies
12.3.2. Biotechnology Companies
12.3.3. Medical Device Companies
12.3.4. Contract Research Organizations (CROs)
12.4. Asia Pacific Recombinant Factor C Assay Market Value Forecast, by Country/Sub-region, 2017–2027
12.4.1. China
12.4.2. India
12.4.3. Japan
12.4.4. Australia & New Zealand
12.4.5. Rest of Asia Pacific
12.5. Asia Pacific Recombinant Factor C Assay Market Attractiveness Analysis
12.5.1. By Brand
12.5.2. By End-user
12.5.3. By Country/Sub-region
13. Latin America Recombinant Factor C Assay Market Analysis and Forecast
13.1. Key Findings
13.2. Latin America Recombinant Factor C Assay Market Value Forecast, by Brand, 2017–2027
13.2.1. PyroGene
13.2.1.1. PyroGene Bulk rFC (2,880 tests)
13.2.1.2. PyroGene rFC (192 tests)
13.2.2. EndoNext
13.2.2.1. Endozyme II
13.2.2.2. Endozyme II GO
13.2.2.3. EndoLisa
13.3. Latin America Recombinant Factor C Assay Market Value Forecast, by End-user, 2017–2027
13.3.1. Pharmaceutical Companies
13.3.2. Biotechnology Companies
13.3.3. Medical Device Companies
13.3.4. Contract Research Organizations (CROs)
13.4. Latin America Recombinant Factor C Assay Market Value Forecast, by Country/Sub-region, 2017–2027
13.4.1. Brazil
13.4.2. Mexico
13.4.3. Rest of Latin America
13.5. Latin America Recombinant Factor C Assay Market Attractiveness Analysis
13.5.1. By Product
13.5.2. By End-user
13.5.3. By Country/Sub-region
14. Middle East & Africa Recombinant Factor C Assay Market Analysis and Forecast
14.1. Key Findings
14.2. Middle East & Africa Recombinant Factor C Assay Market Value Forecast, by Brand, 2017–2027
14.2.1. PyroGene
14.2.1.1. PyroGene Bulk rFC (2,880 tests)
14.2.1.2. PyroGene rFC (192 tests)
14.2.2. EndoNext
14.2.2.1. Endozyme II
14.2.2.2. Endozyme II GO
14.2.2.3. EndoLisa
14.3. Middle East & Africa Recombinant Factor C Assay Market Value Forecast, by End-user, 2017–2027
14.3.1. Pharmaceutical Companies
14.3.2. Biotechnology Companies
14.3.3. Medical Device Companies
14.3.4. Contract Research Organizations (CROs)
14.4. Middle East & Africa Recombinant Factor C Assay Market Value Forecast, by Country/Sub-region, 2017–2027
14.4.1. GCC Countries
14.4.2. South Africa
14.4.3. Rest of Middle East & Africa
14.5. Middle East & Africa Recombinant Factor C Assay Market Attractiveness Analysis
14.5.1. By Product
14.5.2. By End-user
14.5.3. By Country/Sub-region
15. Competition Landscape
15.1. Company Share Analysis
15.2. Company Profiles
15.2.1. Lonza Group
15.2.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.1.2. Financial Overview
15.2.1.3. Product Portfolio
15.2.1.4. Strategic Overview
15.2.1.5. SWOT Analysis
15.2.2. Hyglos GmbH – a bioMérieux Company
15.2.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.2.2. Financial Overview
15.2.2.3. Product Portfolio
15.2.2.4. Strategic Overview
15.2.2.5. SWOT Analysis
List of Tables
Table 01: Patent Information – Pyrogene (Lonza)
Table 02: Patent Information – EndoLisa
Table 03: Patent Information – EndoZyme II GO
Table 04: Patent Information – Recombinant Factor C Assay Market
Table 05: Product Features & USPs – Recombinant Factor C Assays
Table 06: Pyrogen Testing- Service Providers
Table 07: Pyrogen Testing- Service Providers
Table 08: Pyrogen Testing Products Marketing
Table 09: Cost per Assay and Turn Around Time (LAL)
Table 10: Institutes Involved in Research Studies using Horseshoe Crabs
Table 11: Institutes Focused on Conversing Horseshoe Crabs
Table 12: Pyrogen Testing Market Offerings, By Company
Table 13: Global Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Brand, 2017–2027
Table 14: Global Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by PyroGene, 2017–2027
Table 15: Global Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by EndoNext, 2017–2027
Table 16: Global Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by End-user, 2017–2027
Table 17: Global Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Region, 2017–2027
Table 18: North America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Country, 2017–2027
Table 19: North America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Brand, 2017–2027
Table 20: North America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by PyroGene, 2017–2027
Table 21: North America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by EndoNext, 2017–2027
Table 22: North America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by End-user, 2017–2027
Table 23: Europe Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Country/Sub-region, 2017–2027
Table 24: Europe Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Brand, 2017–2027
Table 25: Europe Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by PyroGene, 2017–2027
Table 26: Europe Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by EndoNext, 2017–2027
Table 27: Europe Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by End-user, 2017–2027
Table 28: Asia Pacific Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Country/Sub-region, 2017–2027
Table 29: Asia Pacific Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Brand, 2017–2027
Table 30: Asia Pacific Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by PyroGene, 2017–2027
Table 31: Asia Pacific Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by EndoNext, 2017–2027
Table 32: Asia Pacific Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by End-user, 2017–2027
Table 33: Latin America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Country/Sub-region, 2017–2027
Table 34: Latin America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Brand, 2017–2027
Table 35: Latin America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by PyroGene, 2017–2027
Table 36: Latin America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by EndoNext, 2017–2027
Table 37: Latin America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by End-user, 2017–2027
Table 38: Middle East & Africa Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Country/Sub-region, 2017–2027
Table 39: Middle East & Africa Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by Brand, 2017–2027
Table 40: Middle East & Africa Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by PyroGene, 2017–2027
Table 41: Middle East & Africa Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by EndoNext, 2017–2027
Table 42: Middle East & Africa Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, by End-user, 2017–2027
List of Figures
Figure 01: Global Recombinant Factor C Assay Market Snapshot
Figure 02: Global Recombinant Factor C Assay Market Value (US$ Thousand), by Region, 2018 and 2027
Figure 03: Global Recombinant Factor C Assay Market Value (US$ Thousand) and Y-o-Y (%) Forecast, 2017–2027
Figure 04: Global Recombinant Factor C Assay Market Value Share, by Brand, 2019
Figure 05: Global Recombinant Factor C Assay Market Value Share, by End-user, 2019
Figure 06: Global Recombinant Factor C Assay Market Value Share, by Region, 2019
Figure 07: Value Chain Analysis – Recombinant Factor C Assay Market
Figure 08: Key Industry Events – Recombinant Factor C Assays Market
Figure 09: Regulatory Scenario – North America
Figure 10: Regulatory Scenario – Europe
Figure 11: Regulatory Scenario – Asia Pacific
Figure 12: Global Recombinant Factor C Assay Market Value Share Analysis, by Brand, 2018 and 2027
Figure 13: Global Recombinant Factor C Assay Market Attractiveness Analysis, by Brand, 2019–2027
Figure 14: Global Recombinant Factor C Assay Market Revenue (US$ Thousand) and Y-o-Y Growth (%), by PyroGene, 2017–2027
Figure 15: Global Recombinant Factor C Assay Market Revenue (US$ Thousand) and Y-o-Y Growth (%), by EndoNext, 2017–2027
Figure 16: Key Trends – By Brand
Figure 17: Global Recombinant Factor C Assay Market Value Share Analysis, by End-user, 2018 and 2027
Figure 18: Global Recombinant Factor C Assay Market Attractiveness Analysis, by End-user, 2019–2027
Figure 19: Global Recombinant Factor C Assay Market Revenue (US$ Thousand) and Y-o-Y Growth (%), by Pharmaceutical Companies, 2017–2027
Figure 20: Global Recombinant Factor C Assay Market Revenue (US$ Thousand) and Y-o-Y Growth (%), by Biotechnology Companies, 2017–2027
Figure 21: Global Recombinant Factor C Assay Market Revenue (US$ Thousand) and Y-o-Y Growth (%), by Medical Device Companies, 2017–2027
Figure 22: Global Recombinant Factor C Assay Market Revenue (US$ Thousand) and Y-o-Y Growth (%), by Contract Research Organizations (CROs), 2017–2027
Figure 23: Key Trends – By End-user
Figure 24: Recombinant Factor C Assay Market – Regional Outlook
Figure 25: Global Recombinant Factor C Assay Market Value (US$ Thousand) Forecast, 2017–2027
Figure 26: Global Recombinant Factor C Assay Market Value Share Analysis, by Region, 2018 and 2027
Figure 27: Global Recombinant Factor C Assay Market Attractiveness Analysis, by Region, 2019–2027
Figure 28: North America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast and Y-o-Y Growth (%) Projection, 2017–2027
Figure 29: North America Recombinant Factor C Assay Market Value Share Analysis, by Country, 2018 and 2027
Figure 30: North America Recombinant Factor C Assay Market Attractiveness Analysis, by Country, 2019–2027
Figure 31: North America Recombinant Factor C Assay Market Value Share Analysis, by Brand, 2018 and 2027
Figure 32: North America Recombinant Factor C Assay Market Attractiveness Analysis, by Brand, 2019–2027
Figure 33: North America Recombinant Factor C Assay Market Value Share Analysis, by End-user, 2018 and 2027
Figure 34: North America Recombinant Factor C Assay Market Attractiveness Analysis, by End-user, 2019–2027
Figure 35: Europe Recombinant Factor C Assay Market Value (US$ Thousand) Forecast and Y-o-Y Growth (%) Projection, 2017–2027
Figure 36: Europe Recombinant Factor C Assay Market Value Share Analysis, by Country/Sub-region, 2018 and 2027
Figure 37: Europe Recombinant Factor C Assay Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 38: Europe Recombinant Factor C Assay Market Value Share Analysis, by Brand, 2018 and 2027
Figure 39: Europe Recombinant Factor C Assay Market Attractiveness Analysis, by Brand, 2019–2027
Figure 40: Europe Recombinant Factor C Assay Market Value Share Analysis, by End-user, 2018 and 2027
Figure 41: Europe Recombinant Factor C Assay Market Attractiveness Analysis, by End-user, 2019–2027
Figure 42: Asia Pacific Recombinant Factor C Assay Market Value (US$ Thousand) Forecast and Y-o-Y Growth (%) Projection, 2017–2027
Figure 43: Asia Pacific Recombinant Factor C Assay Market Value Share Analysis, by Country/Sub-region, 2018 and 2027
Figure 44: Asia Pacific Recombinant Factor C Assay Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 45: Asia Pacific Recombinant Factor C Assay Market Value Share Analysis, by Brand, 2018 and 2027
Figure 46: Asia Pacific Recombinant Factor C Assay Market Attractiveness Analysis, by Brand, 2019–2027
Figure 47: Asia Pacific Recombinant Factor C Assay Market Value Share Analysis, by End-user, 2018 and 2027
Figure 48: Asia Pacific Recombinant Factor C Assay Market Attractiveness Analysis, by End-user, 2019–2027
Figure 49: Latin America Recombinant Factor C Assay Market Value (US$ Thousand) Forecast and Y-o-Y Growth (%) Projection, 2017–2027
Figure 50: Latin America Recombinant Factor C Assay Market Value Share Analysis, by Country/Sub-region, 2018 and 2027
Figure 51: Latin America Recombinant Factor C Assay Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 52: Latin America Recombinant Factor C Assay Market Value Share Analysis, by Brand, 2018 and 2027
Figure 53: Latin America Recombinant Factor C Assay Market Attractiveness Analysis, by Brand, 2019–2027
Figure 54: Latin America Recombinant Factor C Assay Market Value Share Analysis, by End-user, 2018 and 2027
Figure 55: Latin America Recombinant Factor C Assay Market Attractiveness Analysis, by End-user, 2019–2027
Figure 56: Middle East & Africa Recombinant Factor C Assay Market Value (US$ Thousand) Forecast and Y-o-Y Growth (%) Projection, 2017–2027
Figure 57: Middle East & Africa Recombinant Factor C Assay Market Value Share Analysis, by Country/Sub-region, 2018 and 2027
Figure 58: Middle East & Africa Recombinant Factor C Assay Market Attractiveness Analysis, by Country/Sub-region, 2019–2027
Figure 59: Middle East & Africa Recombinant Factor C Assay Market Value Share Analysis, by Brand, 2018 and 2027
Figure 60: Middle East & Africa Recombinant Factor C Assay Market Attractiveness Analysis, by Brand, 2019–2027
Figure 61: Middle East & Africa Recombinant Factor C Assay Market Value Share Analysis, by End-user, 2018 and 2027
Figure 62: Middle East & Africa Recombinant Factor C Assay Market Attractiveness Analysis, by End-user, 2019–2027
Figure 63: Global Recombinant Factor C Assay Market Analysis, by Top Company Ranking, 2018