Reports
Reports
Patients with gastrointestinal (GI) diseases may be at increased risk for more severe illness due to the ongoing COVID-19 outbreak. Potential risk factors in these patients include their chronic inflammatory disease, comorbidities, and the use of glucocorticoids. Healthcare, being an essential industry, has compelled manufacturers in the short bowel syndrome market to maintain robust supply chains for treatment options and medicines to reduce morbidity and mortality rates.
The GI tract may be susceptible to coronavirus infection due to the widely expressed angiotensin-converting enzyme 2 (ACE2) receptors in the intestine. Hence, companies in the short bowel syndrome market are increasing the availability of products through online, hospital, and retail pharmacies to meet patient requirements and keep revenue streams flowing during the pandemic.
Today, treating short bowel syndrome (SBS) requires long-term parenteral nutrition or an organ transplant. Since the outcome with the former treatment is uncertain, the latter is linked to the shortage of organs. Thus, researchers with the EU-funded INTENS project are striving to develop a better solution. Companies in the short bowel syndrome market are taking cues from such researchers to develop strategy for autologous tissue engineering, which is known as the process of treating an individual using their own cells or tissues.
The autologous tissue engineering strategy holds promising potentials to overcome the shortage of organs and avoid the need of the risky practice of suppressing the patient’s immune response.
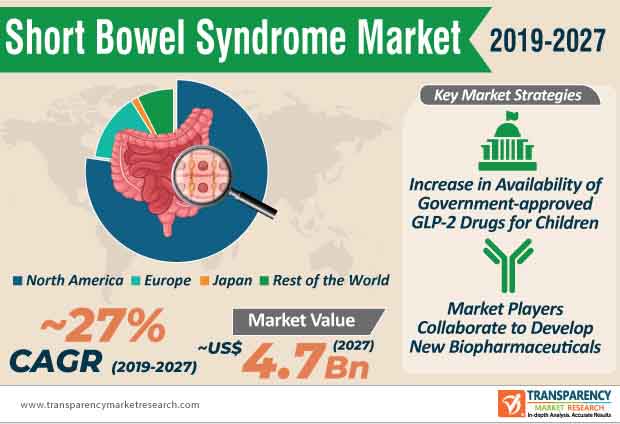
The short bowel syndrome market is estimated to cross US$ 3.66 Bn by 2031. Apart from glucagon-like peptide-2 (GLP2) and growth hormones, companies are boosting their production capabilities in fish oil-based emulsions for patients suffering from intestinal failure-associated liver disease and liver cholestasis.
There is a growing demand for intravenous supplementation of fluids to maintain normal hydration and urinary flow for patients suffering from SBS. Manufacturers in the short bowel syndrome market are increasing efforts to meet this demand, since SBS patients suffer from complications such as dehydration, hyponatremia, and chronic renal failure. Since hypomagnesemia is caused by malabsorption of magnesium due to loss of the distal ileum, manufacturers are producing magnesium supplements to help patients achieve adequate hydration.
Nutritional support, medications, and surgery are the most preferred treatment options for SBS. Companies in the short bowel syndrome market are expanding their revenue streams in oral rehydration products including sports drinks and sodas without caffeine. Children are being recommended special drinks that contain salts and minerals such as Infalyte, CeraLyte, and Pedialyte, which are being made available at drug stores and grocery stores, to prevent dehydration.
Manufacturers in the short bowel syndrome market are establishing stable income sources through prescription medicines such as antibiotics, H2 blockers, proton pump inhibitors, and the likes to improve patient outcomes.
Analysts’ Viewpoint
Since patients with COVID-19 have reported gastrointestinal symptoms, such findings are creating additional revenue opportunities for manufacturers in the short bowel syndrome market. The short bowel syndrome market is expected to grow at a CAGR of 15.9% during the forecast period. However, the SBS treatment requires long-term parenteral nutrition or an organ transplant. Hence, companies should collaborate with researchers to increase the study in autologous tissue engineering, which helps in treating an individual using their own cells or tissues. Companies are boosting their output capacities in anti-secretin agents to help patients reduce gastric acid in the intestine, growth hormones, and hypomotility agents to increase the time it takes the food to travel through intestines, resulting in increased nutrient absorption.
Short bowel syndrome market is estimated to cross US$ 3.66 Bn by 2031
Short bowel syndrome market is projected to expand at a CAGR of 15.9% from 2021 to 2031
Short bowel syndrome market is driven by rise in prevalence of short bowel syndrome, increase in demand for SBS drugs due to high cost of parenteral nutrition, and favorable reimbursement policies
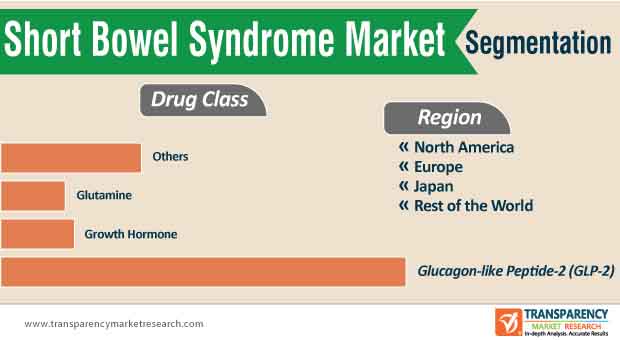
The glucagon-like peptide-2 segment dominated the global short bowel syndrome market in 2020, and the trend is projected to continue during the forecast period
Key players in the global short bowel syndrome market include Takeda Pharmaceutical Company Limited, Merck KGaA, Zealand Pharma A/S, OxThera, VectivBio AG, 9 Meters Biopharma, Inc., Nutrinia Ltd., Hanmi Pharm.Co., Ltd., and Pharmascience, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Short Bowel Syndrome Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Short Bowel Syndrome Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Short Bowel Syndrome: Disease Overview
5.2. Pipeline Analysis & Clinical Trials Analysis
5.3. Disease Prevalence & Incidence Rate, Globally with Key Regions
5.4. Key Industry Events (mergers, acquisitions, collaborations, approvals, etc.)
5.5. COVID-19 Pandemic Impact on Industry
6. Global Short Bowel Syndrome Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value & Volume Forecast, by Product, 2017–2031
6.3.1. Glucagon-like Peptide-2 (GLP2)
6.3.2. Growth Hormone
6.3.3. Glutamine
6.3.4. Others
6.4. Market Attractiveness Analysis, by Product
7. Global Short Bowel Syndrome Market Analysis and Forecast, by Distribution Channel
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value & Volume Forecast, by Distribution Channel, 2017–2031
7.3.1. Hospital Pharmacies
7.3.2. Retail Pharmacies
7.3.3. Online Sales
7.4. Market Attractiveness Analysis, by Distribution Channel
8. Global Short Bowel Syndrome Market Analysis and Forecast, by Region
8.1. Key Findings
8.2. Market Value & Volume Forecast, by Region
8.2.1. North America
8.2.2. Europe
8.2.3. Japan
8.2.4. Australia
8.2.5. Rest Of World
8.3. Market Attractiveness Analysis, by Country/Region
9. North America Short Bowel Syndrome Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value & Volume Forecast, by Product, 2017–2031
9.2.1. Glucagon-like Peptide-2 (GLP2)
9.2.2. Growth Hormone
9.2.3. Glutamine
9.2.4. Others
9.3. Market Value & Volume Forecast, by Distribution Channel, 2017–2031
9.3.1. Hospital Pharmacies
9.3.2. Retail Pharmacies
9.3.3. Online Sales
9.4. Market Value & Volume Forecast, by Country, 2017–2031
9.4.1. U.S.
9.4.2. Canada
9.5. Market Attractiveness Analysis
9.5.1. By Product
9.5.2. By Distribution Channel
9.5.3. By Country
10. Europe Short Bowel Syndrome Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value & Volume Forecast, by Product, 2017–2031
10.2.1. Glucagon-like Peptide-2 (GLP2)
10.2.2. Growth Hormone
10.2.3. Glutamine
10.2.4. Others
10.3. Market Value & Volume Forecast, by Distribution Channel, 2017–2031
10.3.1. Hospital Pharmacies
10.3.2. Retail Pharmacies
10.3.3. Online Sales
10.4. Market Value & Volume Forecast, by Country/Sub-Region, 2017–2031
10.4.1. Germany
10.4.2. U.K.
10.4.3. France
10.4.4. Spain
10.4.5. Italy
10.4.6. Rest of Europe
10.5. Market Attractiveness Analysis
10.5.1. By Product
10.5.2. By Distribution Channel
10.5.3. By Country/Sub-Region
11. Japan Short Bowel Syndrome Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value & Volume Forecast, by Product, 2017–2031
11.2.1. Glucagon-like Peptide-2 (GLP2)
11.2.2. Growth Hormone
11.2.3. Glutamine
11.2.4. Others
11.3. Market Value & Volume Forecast, by Distribution Channel, 2017–2031
11.3.1. Hospital Pharmacies
11.3.2. Retail Pharmacies
11.3.3. Online Sales
11.4. Market Attractiveness Analysis
11.4.1. By Product
11.4.2. By Distribution Channel
12. Australia Short Bowel Syndrome Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value & Volume Forecast, by Product, 2017–2031
12.2.1. Glucagon-like Peptide-2 (GLP2)
12.2.2. Growth Hormone
12.2.3. Glutamine
12.2.4. Others
12.3. Market Value & Volume Forecast, by Distribution Channel, 2017–2031
12.3.1. Hospital Pharmacies
12.3.2. Retail Pharmacies
12.3.3. Online Sales
12.4. Market Attractiveness Analysis
12.4.1. By Product
12.4.2. By Distribution Channel
13. Rest Of World Short Bowel Syndrome Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value & Volume Forecast, by Product, 2017–2031
13.2.1. Glucagon-like Peptide-2 (GLP2)
13.2.2. Growth Hormone
13.2.3. Glutamine
13.2.4. Others
13.3. Market Value & Volume Forecast, by Distribution Channel, 2017–2031
13.3.1. Hospital Pharmacies
13.3.2. Retail Pharmacies
13.3.3. Online Sales
13.4. Market Attractiveness Analysis
13.4.1. By Product
13.4.2. By Distribution Channel
14. Competition Landscape
14.1. Market Player – Competition Matrix (by tier and size of companies)
14.2. Market Share Analysis By Company (2020)
14.3. Company Profiles
14.3.1. Takeda Pharmaceutical Company Limited
14.3.1.1. Company Description
14.3.1.2. Business Overview
14.3.1.3. Financial Overview
14.3.1.4. Strategic Overview
14.3.1.5. SWOT Analysis
14.3.2. Merck KGaA
14.3.2.1. Company Description
14.3.2.2. Business Overview
14.3.2.3. Financial Overview
14.3.2.4. Strategic Overview
14.3.2.5. SWOT Analysis
14.3.3. Zealand Pharma A/S
14.3.3.1. Company Description
14.3.3.2. Business Overview
14.3.3.3. Financial Overview
14.3.3.4. Strategic Overview
14.3.3.5. SWOT Analysis
14.3.4. OxThera
14.3.4.1. Company Description
14.3.4.2. Business Overview
14.3.4.3. Financial Overview
14.3.4.4. Strategic Overview
14.3.4.5. SWOT Analysis
14.3.5. VectivBio AG
14.3.5.1. Company Description
14.3.5.2. Business Overview
14.3.5.3. Financial Overview
14.3.5.4. Strategic Overview
14.3.5.5. SWOT Analysis
14.3.6. 9 Meters Biopharma, Inc.
14.3.6.1. Company Description
14.3.6.2. Business Overview
14.3.6.3. Financial Overview
14.3.6.4. Strategic Overview
14.3.6.5. SWOT Analysis
14.3.7. Nutrinia Ltd.
14.3.7.1. Company Description
14.3.7.2. Business Overview
14.3.7.3. Financial Overview
14.3.7.4. Strategic Overview
14.3.7.5. SWOT Analysis
14.3.8. Hanmi Pharm Co., Ltd.
14.3.8.1. Company Description
14.3.8.2. Business Overview
14.3.8.3. Financial Overview
14.3.8.4. Strategic Overview
14.3.8.5. SWOT Analysis
14.3.9. Pharmascience, Inc.
14.3.9.1. Company Description
14.3.9.2. Business Overview
14.3.9.3. Financial Overview
14.3.9.4. Strategic Overview
14.3.9.5. SWOT Analysis
List of Tables
Table 01: Global Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 02: Global Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 03: Global Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 04: North America Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 05: North America Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 06: North America Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 07: Europe Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Country/Sub-Region, 2017–2031
Table 08: Europe Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 09: Europe Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 10: Japan Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 11: Japan Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 12: Australia Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 13: Australia Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 14: Rest of the World Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 15: Rest of the World Short Bowel Syndrome Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
List of Figures
Figure 01: Short Bowel Syndrome Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Short Bowel Syndrome Market Value Share, by Product, 2020
Figure 03: Short Bowel Syndrome Market Value Share, by Distribution Channel, 2020
Figure 04: Global Short Bowel Syndrome Market Value Share Analysis, by Product, 2020 and 2031
Figure 05: Global Short Bowel Syndrome Market Attractiveness Analysis, by Product, 2021–2031
Figure 06: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Glucagon-like Peptide-2 (GLP2), 2017–2031
Figure 07: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Growth Hormone, 2017–2031
Figure 08: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Glutamine, 2017–2031
Figure 09: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Others, 2017–2031
Figure 10: Global Short Bowel Syndrome Market Value Share Analysis, by Distribution Channel, 2020 and 2031
Figure 11: Global Short Bowel Syndrome Market Attractiveness Analysis, by Distribution Channel, 2021–2031
Figure 12: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Hospital Pharmacies, 2017–2031
Figure 13: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Retail Pharmacies, 2017–2031
Figure 14: Global Short Bowel Syndrome Market Revenue (US$ Mn), by Online Sales, 2017–2031
Figure 15: Global Short Bowel Syndrome Market Value Share Analysis, by Region, 2020 and 2031
Figure 16: Global Short Bowel Syndrome Market Attractiveness Analysis, by Region, 2021–2031
Figure 17: North America Short Bowel Syndrome Market Value (US$ Mn) Forecast, 2017–2031
Figure 18: North America Short Bowel Syndrome Market Value Share Analysis, by Country, 2020 and 2031
Figure 19: North America Short Bowel Syndrome Market Attractiveness Analysis, by Country, 2021–2031
Figure 20: North America Short Bowel Syndrome Market Value Share Analysis, by Product, 2020 and 2031
Figure 21: North America Short Bowel Syndrome Market Attractiveness Analysis, by Product, 2021–2031
Figure 22: North America Short Bowel Syndrome Market Value Share Analysis, by Distribution Channel, 2020 and 2031
Figure 23: North America Short Bowel Syndrome Market Attractiveness Analysis, by Distribution Channel, 2021–2031
Figure 24: Europe Short Bowel Syndrome Market Value (US$ Mn) Forecast, 2017–2031
Figure 25: Europe Short Bowel Syndrome Market Value Share Analysis, by Country/Sub-Region, 2020 and 2031
Figure 26: Europe Short Bowel Syndrome Market Attractiveness Analysis, by Country/Sub-region, 2021–2031
Figure 27: Europe Short Bowel Syndrome Market Value Share Analysis, by Product, 2020 and 2031
Figure 28: Europe Short Bowel Syndrome Market Attractiveness Analysis, by Product, 2021–2031
Figure 29: Europe Short Bowel Syndrome Market Value Share Analysis, by Distribution Channel, 2020 and 2031
Figure 30: Europe Short Bowel Syndrome Market Attractiveness Analysis, by Distribution Channel, 2021–2031
Figure 31: Japan Short Bowel Syndrome Market Value (US$ Mn) Forecast, 2017–2031
Figure 32: Japan Short Bowel Syndrome Market Value Share Analysis, by Product, 2020 and 2031
Figure 33: Japan Short Bowel Syndrome Market Attractiveness Analysis, by Product, 2021–2031
Figure 34: Japan Short Bowel Syndrome Market Value Share Analysis, by Distribution Channel, 2020 and 2031
Figure 35: Japan Short Bowel Syndrome Market Attractiveness Analysis, by Distribution Channel, 2021–2031
Figure 36: Australia Short Bowel Syndrome Market Value (US$ Mn) Forecast, 2017–2031
Figure 37: Australia Short Bowel Syndrome Market Value Share Analysis, by Product, 2020 and 2031
Figure 38: Australia Short Bowel Syndrome Market Attractiveness Analysis, by Product, 2021–2031
Figure 39: Australia Short Bowel Syndrome Market Value Share Analysis, by Distribution Channel, 2020 and 2031
Figure 40: Australia Short Bowel Syndrome Market Attractiveness Analysis, by Distribution Channel, 2021–2031
Figure 41: Rest of the World Short Bowel Syndrome Market Value (US$ Mn) Forecast, 2017–2031
Figure 42: Rest of the World Short Bowel Syndrome Market Value Share Analysis, by Product, 2020 and 2031
Figure 43: Rest of the World Short Bowel Syndrome Market Attractiveness Analysis, by Product, 2021–2031
Figure 44: Rest of the World Short Bowel Syndrome Market Value Share Analysis, by Distribution Channel, 2020 and 2031
Figure 45: Rest of the World Short Bowel Syndrome Market Attractiveness Analysis, by Distribution Channel, 2021–2031