Reports
Reports
Analysts’ Viewpoint on Point-of-Care Coagulation Testing Devices Market Scenario
Increase in point-of-care coagulation testing (POCT) in developed countries and rise in prevalence of blood clotting disorders are driving the global point-of-care coagulation testing devices market. Various point-of-care (POC) assays are available for a number of coagulation tests. These assays are easy to perform and have a faster turnaround time than their laboratory counterparts. Portability, easy usage, and connectivity are propelling the demand for POC coagulation testing procedures. North America and Europe are likely to be major markets for manufacturers in the near future, owing to the availability of low-cost devices, increase in chronic diseases, and rise in popularity of portable testing equipment in these regions.
Coagulation, or clotting, is the process of conversion of liquid blood into a gel-like clot to stop bleeding. The capability of the body cells to prevent blood loss after a vascular injury by forming a blood clot is vital to sustaining health. A number of coagulation or clotting factors are involved that lead to a physiological procedure called hemostasis. Hemostasis is the mechanism of clotting that involves activation, adhesion, and aggregation of platelets mediated by several clotting factors, eventually leading to the repair of the injured tissue. The absence of any of these factors could lead to a rare, but serious blood clotting disease and excessive bleeding. Point-of-care testing (POCT) can be defined as a fast, specific medical diagnostic testing of bodily fluids at the bedside. It is often termed as decentralized diagnostic testing.
Rise in prevalence of blood clotting disorders, increase in POCT coagulation in developed countries, and surge in usage of anticoagulant therapy are the factors driving the global POC coagulation testing devices market. Furthermore, increase in prevalence of cardiovascular diseases & cancer, constant product & technology innovations, and funding by the government are propelling the global market. However, lack of awareness in developing countries and regulatory challenges are projected to restrain the global point-of-care coagulation testing devices market. A number of studies have shown that 60% to 70% of deep vein thrombosis in developing countries is clinically undiagnosed. Nevertheless, players in the point-of-care coagulation devices market are establishing a stronger foothold in developed and emerging markets.
The COVID-19 pandemic has had a negative impact on the global point-of-care coagulation testing devices market. Several affected countries deferred non-essential surgeries and patient visits during the breakout year of the pandemic. This led to a substantial decline in overall non-emergency surgery and treatments, thereby limiting the usage of point-of-care coagulation testing instruments during preoperative or post-operative conditions. The pandemic caused a decline in key players’ revenue. However, demand for vital function monitoring viscoelastic devices, including venous thromboembolism (VTE) and Thromboelastography (TEG), has increased during the pandemic, as these devices help detect pulmonary embolism (PE) and venous thromboembolism (VTE) among critically ill COVID-19 patients. According to the Intensive Care Medicine Journal, in May 2020, the incidence of prothrombotic conditions in patients with severe symptoms of COVID-19 infection increased significantly. This resulted in a rise in number of coagulation tests such as prothrombin time (PT), D-dimer, TEG, and activated partial thromboplastin time (aPTT).
In terms of product, the consumables & accessories segment accounted for major share of the global point-of-care coagulation testing devices market in 2021. Increase in usage of kits & reagents, which provide results in less time; growth in usage of POC testing strips that provide reliable results; rise in demand for quicker results; and surge in POC testing are the major factors augmenting the consumables & accessories segment. Rapid coagulation testing methods are increasingly adopted by patients. Moreover, adoption of POC coagulation analyzers is projected to rise in the near future, which is likely to drive the segment. The usage of disposable consumables is increasing due to the rise in awareness about the prevention of blood-borne diseases, such as hepatitis B and HIV, among the people. Thus, assurance of patient safety due to usage of disposable consumables and reliable results is propelling the segment.
The PT/INR segment held the largest share of the market in terms of revenue in 2021. The segment is anticipated to dominate the global market owing to the growing trend of point-of-care testing and self-monitoring with PT tests. Moreover, high reliability of the coagulation status on the PT test is one of the factors likely to drive the segment during the forecast period. Key players are focused on the launch of technologically advanced products. For instance, Roche developed a Bluetooth-enabled PT/INR testing CoaguChek device that helps patients and health care providers to check coagulation status and help in remote care. Similarly, Siemens Healthineers launched the FDA-approved POC PT/INR device called Xprecia Stride Coagulation Analyzer. This device has demonstrated PT/INR testing performance equivalency with the Roche CoaguChek XS POC system.
The clinics segment is anticipated to grow at a rapid pace during the forecast period. Factors such as faster results, convenience of testing, prevalence of chronic diseases, and preoperative testing are attributed to the growth of the clinics segment. According to a report, in 2018, around 129 million adults in the U.S. had been diagnosed with at least one chronic condition; 61 million adults among them had one chronic condition, while 68 million had more than two chronic conditions. Furthermore, rise in number of private clinics and increase in skilled personnel to perform the tests are the major factors augmenting the clinics segment. For instance, India had an estimated 69,000 private and public clinics in 2019. Similarly, China had more than 50,000 private and public clinics.
North America is projected to be a highly attractive region of the global point-of-care coagulation testing devices market during the forecast period. The region accounted for the largest share of the global market in 2021. High prevalence of cardiac diseases, technologically advanced countries, and rise in awareness about coagulation disorders are factors propelling the market in North America. Recent FDA approvals for point-of-care coagulation testing devices such as Abbott's i-STAT indicate increasing demand and support among the public and private sectors along with manufacturers’ focus to capture this rising demand. The U.S. accounted for large share of the market in North America due to the rise in cases of chronic diseases and surge in uptake of early disease detection procedures in the country.
The market in Asia Pacific is likely to grow at the fastest CAGR from 2022 to 2031. The region is witnessing gradual adoption of novel products, increase in health care expenditure, and changes in dynamics in the in vitro diagnostics industry. This is expected to drive the adoption of new technologies among hospitals and diagnostic laboratories.
The point-of-care coagulation testing devices market report includes vital information about the key players operating in the global market. Prominent players are focusing on strategies such as new product launches, divestiture, mergers & acquisitions (M&A), and partnerships to strengthen their position in the market. Teleflex Incorporated, F. Hoffmann-La Roche AG, Abbott Laboratories, Siemens Healthineers, Medtronic plc, Haemonetics Corporation, HemoSonics, LLC, Micropoint Bioscience, Inc., Werfen, Sienco, Inc., and Koninklijke Philips N.V. are some of the key players operating in the global market.
Each of these players has been profiled in the global point-of-care coagulation testing devices market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Market Size Value in 2021 |
US$ 1.69 Bn |
|
Market Forecast Value in 2031 |
More than US$ 3.06 Bn |
|
Compound Annual Growth Rate (CAGR) |
5.4% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2021 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
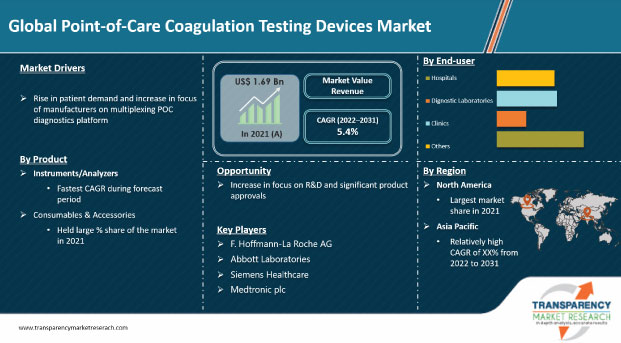
The global point-of-care coagulation testing devices market was valued at US$ 1.69 Bn in 2021
The global market is projected to reach more than US$ 3.06 Bn by 2031
The global point-of-care coagulation testing devices market grew at a CAGR of 2.53% from 2017 to 2021
The global point-of-care coagulation testing devices market is anticipated to grow at a CAGR of 5.4% from 2022 to 2031
Rise in patient demand and increase in focus of manufacturers on multiplexing POC diagnostics platform are driving the global point-of-care coagulation testing devices market
The PT/INR segment held the largest share of around 70% of the market in terms of revenue in 2021
North America is expected to account for major share of the global point-of-care coagulation testing devices market during the forecast period
F. Hoffmann-La Roche AG, Abbott Laboratories, Siemens Healthineers, Medtronic plc, Haemonetics Corporation, HemoSonics, LLC, Micropoint Bioscience, Inc., Werfen, Sienco, Inc., and Koninklijke Philips N.V.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Point-of-Care Coagulation Testing Devices Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Point-of-Care Coagulation Testing Devices Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Technological Advancements
5.2. Key Mergers & Acquisitions
5.3. COVID-19 Pandemics Impact on Industry (value chain and short / mid / long term impact)
6. Global Point-of-Care Coagulation Testing Devices Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Product, 2017–2031
6.3.1. Instruments / Analyzers
6.3.2. Consumables & Accessories
6.4. Market Attractiveness Analysis, by Product
7. Global Point-of-Care Coagulation Testing Devices Market Analysis and Forecast, by Method
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Method, 2017–2031
7.3.1. PT/INR
7.3.2. Viscoelastic Coagulation Monitoring
7.3.3. Others (ACT, aPTT, etc.)
7.4. Market Attractiveness Analysis, by Method
8. Global Point-of-Care Coagulation Testing Devices Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by End-user, 2017–2031
8.3.1. Hospitals
8.3.2. Diagnostic Laboratories
8.3.3. Clinics
8.4. Market Attractiveness Analysis, by End-user
9. Global Point-of-Care Coagulation Testing Devices Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Point-of-Care Coagulation Testing Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Product, 2017–2031
10.2.1. Instruments / Analyzers
10.2.2. Consumables & Accessories
10.3. Market Value Forecast, by Method, 2017–2031
10.3.1. PT/INR
10.3.2. Viscoelastic Coagulation Monitoring
10.3.3. Others (ACT, aPTT, etc.)
11. Europe Point-of-Care Coagulation Testing Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Product, 2017–2031
11.2.1. Instruments / Analyzers
11.2.2. Consumables & Accessories
11.3. Market Value Forecast, by Method, 2017–2031
11.3.1. PT/INR
11.3.2. Viscoelastic Coagulation Monitoring
11.3.3. Others (ACT, aPTT, etc.)
11.4. Market Value Forecast, by End-user, 2017–2031
11.4.1. Hospitals
11.4.2. Diagnostic Laboratories
11.4.3. Clinics
11.4.4. Others
11.5. Market Value Forecast, by Country/Sub-region, 2017–2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Product
11.6.2. By Method
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Point-of-Care Coagulation Testing Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Product, 2017–2031
12.2.1. Instruments / Analyzers
12.2.2. Consumables & Accessories
12.3. Market Value Forecast, by Method, 2017–2031
12.3.1. PT/INR
12.3.2. Viscoelastic Coagulation Monitoring
12.3.3. Others (ACT, aPTT, etc.)
12.4. Market Value Forecast, by End-user, 2017–2031
12.4.1. Hospitals
12.4.2. Diagnostic Laboratories
12.4.3. Clinics
12.4.4. Others
12.5. Market Value Forecast, by Country/Sub-region, 2017–2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Product
12.6.2. By Method
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America Point-of-Care Coagulation Testing Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Product, 2017–2031
13.2.1. Instruments / Analyzers
13.2.2. Consumables & Accessories
13.3. Market Value Forecast, by Method, 2017–2031
13.3.1. PT/INR
13.3.2. Viscoelastic Coagulation Monitoring
13.3.3. Others (ACT, aPTT, etc.)
13.4. Market Value Forecast, by End-user, 2017–2031
13.4.1. Hospitals
13.4.2. Diagnostic Laboratories
13.4.3. Clinics
13.4.4. Others
13.5. Market Value Forecast, by Country/Sub-region, 2017–2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Product
13.6.2. By Method
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Point-of-Care Coagulation Testing Devices Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Product, 2017–2031
14.2.1. Instruments / Analyzers
14.2.2. Consumables & Accessories
14.3. Market Value Forecast, by Method, 2017–2031
14.3.1. PT/INR
14.3.2. Viscoelastic Coagulation Monitoring
14.3.3. Others (ACT, aPTT, etc.)
14.4. Market Value Forecast, by End-user, 2017–2031
14.4.1. Hospitals
14.4.2. Diagnostic Laboratories
14.4.3. Clinics
14.4.4. Others
14.5. Market Value Forecast, by Country/Sub-region, 2017–2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Product
14.6.2. By Method
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competition Matrix (by tier and size of companies)
15.2. Company Profiles
15.3. Company Profiles
15.3.1. F. Hoffmann-La Roche AG
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. SWOT Analysis
15.3.1.4. Strategic Overview
15.3.2. Abbott Laboratories
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. SWOT Analysis
15.3.2.5. Strategic Overview
15.3.3. Siemens Healthineers
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. SWOT Analysis
15.3.3.4. Strategic Overview
15.3.4. Medtronic plc
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. SWOT Analysis
15.3.4.5. Strategic Overview
15.3.5. Haemonetics Corporation
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. SWOT Analysis
15.3.5.5. Strategic Overview
15.3.6. HemoSonics, LLC
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. SWOT Analysis
15.3.6.4. Strategic Overview
15.3.7. Micropoint Bioscience, Inc.
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. SWOT Analysis
15.3.7.5. Strategic Overview
15.3.8. Werfen
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Financial Overview
15.3.8.4. SWOT Analysis
15.3.8.5. Strategic Overview
15.3.9. Sienco, Inc.
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. SWOT Analysis
15.3.9.4. Strategic Overview
15.3.10. Koninklijke Philips N.V.
15.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.10.2. Product Portfolio
15.3.10.3. SWOT Analysis
15.3.10.4. Strategic Overview
List of Tables
Table 01: Global Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Product, 2022-2031
Table 02: Global Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Method, 2022-2031
Table 03: Global Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by End-user, 2022-2031
Table 04: Global Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Region, 2022-2031
Table 05: North America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Product, 2022-2031
Table 06: North America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Method, 2022-2031
Table 07: North America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by End-user, 2022-2031
Table 08: North America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Country, 2022-2031
Table 09: Europe Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Product, 2022-2031
Table 10: Europe Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Method, 2022-2031
Table 11: Europe Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by End-user, 2022-2031
Table 12: Europe Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2022-2031
Table 13: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Product
Table 14: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Method
Table 15: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by End-user
Table 16 Asia Pacific Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Country/Sub-region
Table 17: Latin America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Product
Table 18: Latin America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Method, 2022-2031
Table 19: Latin America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by End-user, 2022-2031
Table 20: Latin America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2022-2031
Table 21: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Product, 2022-2031
Table 22: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Method, 2022-2031
Table 23: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by End-user, 2022-2031
Table 24: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2022-2031
List of Figures
Figure 01: Global Point-of-Care Coagulation Testing Devices Market Size (US$ Mn) Forecast 2022-2031
Figure 02: Global Point-of-Care Coagulation Testing Devices Market Value Share, by Method, 2022
Figure 03: Global Point-of-Care Coagulation Testing Devices Market Value Share, by Product, 2022
Figure 04: Global Point-of-Care Coagulation Testing Devices Market Value Share, by End-user, 2022
Figure 05: Global Point-of-Care Coagulation Testing Devices Market Value Share, by Region, 2022
Figure 06: Global Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 07: Global Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 08: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) by Instruments/Analyzers, 2022-2031
Figure 09: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) by Consumables & Accessories, 2022-2031
Figure 10: Global Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Method, 2021 and 2031
Figure 11: Global Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Method, 2022–2031
Figure 12: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) Forecast, by PT/INR, 2022-2031
Figure 13: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) Forecast, by Others, 2022-2031
Figure 14: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) Forecast, by Viscoelastic Coagulation Monitoring, 2022-2031
Figure 15: Global Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 16: Global Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 17: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) by Hospitals, 2022-2031
Figure 18: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) by Diagnostic Laboratories, 2022-2031
Figure 19: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) by Clinics, 2022-2031
Figure 20: Global Point-of-Care Coagulation Testing Devices Market Revenue (US$ Mn) by Other End-users, 2022-2031
Figure 21: Global Point-of-Care Coagulation Testing Devices Market Value Share, by Region, 2021 and 2031
Figure 22: Global Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Region, 2022–2031
Figure 23: North America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast 2022-2031
Figure 24: North America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 25: North America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 26: North America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Method, 2021 and 2031
Figure 27: North America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Method, 2022–2031
Figure 28: North America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 29: North America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 30: North America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Country, 2021 and 2031
Figure 31: North America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Country, 2022–2031
Figure 32: Europe Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast 2022-2031
Figure 33: Europe Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 34: Europe Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 35: Europe Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Method, 2021 and 2031
Figure 36: Europe Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Method, 2022–2031
Figure 37: Europe Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 38: Europe Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 39: Europe Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 40: Europe Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 41: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast 2022-2031
Figure 42: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 43: Asia Pacific Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 44: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Method, 2021 and 2031
Figure 45: Asia Pacific Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Method, 2022–2031
Figure 46: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 47: Asia Pacific Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 48: Asia Pacific Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 49: Asia Pacific Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 50: Latin America Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast 2022-2031
Figure 51: Latin America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 52: Latin America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 53: Latin America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Method, 2021 and 2031
Figure 54: Latin America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Method, 2022–2031
Figure 55: Latin America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 56: Latin America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 57: Latin America Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 58: Latin America Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 59: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value (US$ Mn) Forecast 2022-2031
Figure 60: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Product, 2021 and 2031
Figure 61: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Product, 2022–2031
Figure 62: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Method, 2021 and 2031
Figure 63: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Method, 2022–2031
Figure 64: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by End-user, 2021 and 2031
Figure 65: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 66: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Value Share Analysis, by Country/Sub-region
Figure 67: Middle East & Africa Point-of-Care Coagulation Testing Devices Market Attractiveness Analysis, by Country/Sub-region
Figure 68: Global Point-of-Care Coagulation Testing Devices Market Analysis, by Top Company Ranking, 2022
Figure 69: F. Hoffmann-La Roche AG Revenue (US$ Bn)
Figure 70: F. Hoffmann-La Roche AG R&D Expenses
Figure 71: F. Hoffmann-La Roche AG Breakdown of Net Sales, by Business Division
Figure 72: F. Hoffmann-La Roche AG Breakdown of Net Sales, by Country/Region
Figure 73: Abbott Laboratories Revenue (US$ Bn)
Figure 74: Abbott Laboratories R&D Intensity and Sales & Marketing (%),
Figure 75: Abbott Laboratories Breakdown of Net Sales, by Business Division
Figure 76: Abbott Laboratories Breakdown of Net Sales, by Country/Region
Figure 77: Abbott Laboratories Revenue (US$ Mn)
Figure 78: Abbott Laboratories R&D Expenses (US$ Mn)
Figure 79: Abbott Laboratories Breakdown of Net Sales, by Business Division
Figure 80: Abbott Laboratories Breakdown of Net Sales, by Country/Region
Figure 81: Medtronic plc Revenue (US$ Mn)
Figure 82: Medtronic plc Breakdown of Net Sales (%), by Region
Figure 83: Medtronic plc R&D Expenditure (US$ Mn)
Figure 84: Medtronic plc Breakdown of Net Sales (%), by Business Segment
Figure 85: Haemonetics Corporation Revenue (US$ Mn)
Figure 86: Haemonetics Corporation R&D Expenses (US$ Mn)
Figure 87: Haemonetics Corporation Net Sales Breakdown (%), by Region
Figure 88: Haemonetics Corporation Net Sales Breakdown (%), by Business Segment
Figure 89: Werfen Revenue (US$ Mn)
Figure 90: Werfen Breakdown of Net Sales (%), by Region
Figure 91: Werfen Breakdown of Net Sales (%), by Business Segment
Figure 92: Koninklijke Philips N.V. Revenue (US$ Mn)
Figure 93: Koninklijke Philips N.V. Breakdown of Net Sales (%), by Region
Figure 94: Koninklijke Philips N.V. R&D Expenditure (US$ Mn)
Figure 95: Koninklijke Philips N.V. Breakdown of Net Sales (%), by Business Segment