Reports
Reports
Analysts’ Viewpoint on Intraosseous Devices Market Scenario
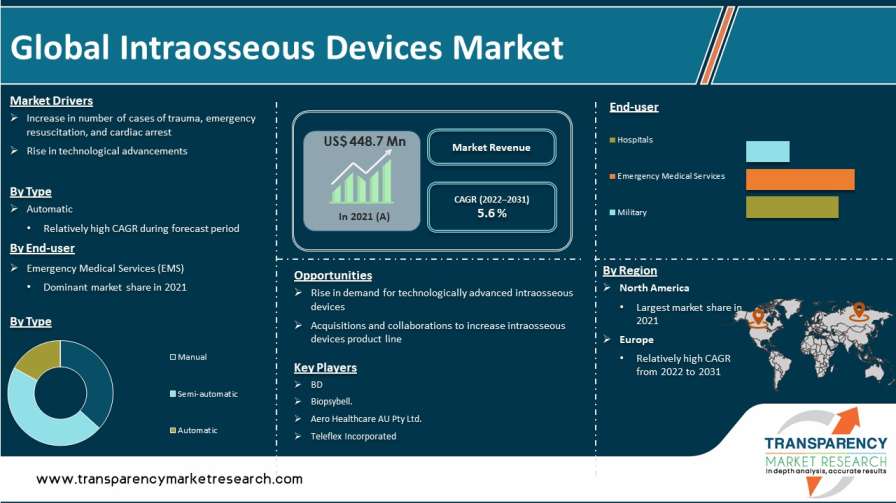
Intraosseous devices or intraosseous infusions can be used to deliver medications and fluids in an emergency situation where venous access is unavailable or cannot be quickly established. Intraosseous devices may help patients receive fluids and treatments through the bone marrow in a safe, effective, and practical manner. The global intraosseous devices market is growing at a steady pace due to the increase in geriatric population, rise in prevalence of various diseases, surge in research activities, rise in number of medical emergency situations, and increase in developmental activities and approaches in the healthcare sector in developed and developing countries. Growth in number of emergency cases, such as heart attacks and trauma-related attacks, is expected to propel the global intraosseous devices market in the next few years.
Medical professionals and trained staff are required to use intraosseous devices as a reliable backup when intravenous access is not possible. Intravenous access in children is challenging and slow. Hence, intraosseous route has emerged as the preferred route in pediatric resuscitation. The device is easily inserted into the medullary space of bone, and the flow of intravenous fluid into the device indicates accurate positioning. Manual needles were the first intraosseous devices to be introduced, which are still extensively used by several practitioners. Several different manual intraosseous needles are commercially available in the market. Recent developments in the intraosseous devices market suggest that manufacturers are investing significantly in research and development activities in order to introduce effective products that increase the efficiency of the therapeutic process. The global intraosseous devices market is driven by improvements in needle tips, new needle assembly, and catheter improvements. Cutting-edge designs and usage of premium materials are also propelling the global intraosseous devices market.
Value-added features offered by intraosseous devices in order to provide effective and efficient vascular access, resulting in positive outcomes and medical aid and care, are expected to drive the global intraosseous device market size in the near future. Intraosseous devices are used to provide effective and quick treatment in different medical situations, particularly emergencies. Medical professionals and trained personnel must use intraosseous devices as an effective alternative when intravenous access is not possible. Companies are strengthening their research & developmental activities and expanding their product lines in order to offer value-added features for the future market demand for intraosseous devices.
Rise in prevalence of trauma cases, emergency resuscitation, and cardiac arrests has increased the patient pool requiring intraosseous access across the world, resulting in high demand for intraosseous devices. According to Heart Disease and Stroke Statistics - 2017 Update by American Heart Association, more than 350,000 out-of-hospital cardiac arrests (OHCA) are reported in the U.S. annually; around 90% of these result in death. Furthermore, in 2015, the annual incidence of EMS-assessed non-traumatic OHCA stood at around 356,500. Moreover, 7,037 children suffer OHCA each year.
In terms of type, the global intraosseous devices market has been classified into manual, semi-automatic, and automatic. Semi-automatic intraosseous devices are more user-friendly than manual devices, and also provide quicker and easier vascular access. Penetration of semi-automatic devices is higher than automatic intraosseous devices. Semi-automatic intraosseous devices provide a wider range of products as compared to manual and automatic intraosseous devices. Demand for semi-automatic devices has increased owing to the availability of more options in the market.
Based on end-user, the global intraosseous market has been classified into hospitals, emergency medical services, and military. The emergency medical services (EMS) segment held the largest share of the global intraosseous devices market in 2021. The segment is expected to grow at a rapid pace during the forecast period. Rise in number of road accidents, trauma cases, cardiac arrests, and other disorders is projected to increase the number of emergency medical situations. This, in turn, is anticipated to augment the EMS segment. High prevalence of diseases, particularly pediatric diseases, and surge in the patient population undergoing emergency situations lead to an increase in the usage of intraosseous devices to provide emergency medical services (EMS).
North America was a highly attractive market for intraosseous devices in 2021. The market in the region is projected to grow at a high CAGR during the forecast period. Highest incidence and prevalence of emergency medical cases and presence of key players in the U.S. are the major factors propelling the intraosseous devices market in the region.
The intraosseous devices market in Europe is expected to grow at a moderate pace during the forecast period. The market in the region is driven by rise in incidence of medical emergency cases and increase in geriatric population. The European Resuscitation Council (ERC) recommends intraosseous access devices during CPR if peripheral vein access is not immediately available. Intraosseous access is a widely accepted method of gaining venous access during a critically ill child's cardiopulmonary resuscitation (CPR).
The market in Asia Pacific is expected to experience notable growth during the forecast period. Increase in access to health care services through non-profit organizations in collaboration with governments, rapid introduction of advanced technological systems in developing countries, and rise in frequency of emergency medical situations are driving the intraosseous devices market in the region. Growth in prevalence of traffic accidents, trauma cases, and other medical emergencies is propelling the intraosseous devices market in Latin America.
The global intraosseous devices market is fragmented, with the presence of many large-scale players. Key players operating in the global intraosseous devices market are Aero Healthcare AU Pty Ltd., Becton, Dickinson and Company (BD), Biopsybell, Cook, PAVmed, Inc., PERSYS MEDICAL, and Teleflex Incorporated. These players are adopting growth strategies such as new product development, product launches, product approval, agreement, partnerships, and mergers.
Leading players have been profiled in the intraosseous devices market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Market Size Value in 2021 |
US$ 448.7 Mn |
|
Market Forecast Value in 2031 |
More than US$ 778.9 Mn |
|
Growth Rate (CAGR) 2022-2031 |
5.6% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2021 |
|
Quantitative Units |
US$ Mn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global intraosseous devices market was valued at US$ 448.7 Mn in 2021
The global intraosseous devices market is projected to reach more than US$ 778.9 Mn by 2031
The global intraosseous devices market grew at a CAGR of 6% from 2017 to 2021
The global intraosseous devices market is anticipated to grow at a CAGR of 5.6% from 2022 to 2031
The automatic segment held major share of around 45% of the global intraosseous devices market in 2021
North America is expected to account for major share of the global intraosseous devices market during the forecast period.
Aero Healthcare AU Pty Ltd., Becton, Dickinson and Company, Biopsybell, Cook, and Teleflex Incorporated
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Intraosseous Devices Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Intraosseous Devices Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Technological Advancements
5.2. Regulatory Scenario by Region/globally
5.3. Covid-19 Impact Analysis
6. Global Intraosseous Devices Market Analysis and Forecast, by Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Type, 2017–2031
6.3.1. Manual
6.3.2. Semi-automatic
6.3.3. Automatic
6.4. Market Attractiveness Analysis, by Type
7. Global Intraosseous Devices Market Analysis and Forecast, by End-user
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by End-user, 2017–2031
7.3.1. Hospitals
7.3.2. Emergency Medical Services
7.3.3. Military
7.4. Market Attractiveness Analysis, by End-user
8. Global Intraosseous Devices Market Analysis and Forecast, by Region
8.1. Key Findings
8.2. Market Value Forecast, by Region
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Latin America
8.2.5. Middle East & Africa
8.3. Market Attractiveness Analysis, by Region
9. North America Intraosseous Devices Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value Forecast, by Type, 2017–2031
9.2.1. Manual
9.2.2. Semi-automatic
9.2.3. Automatic
9.3. Market Value Forecast, by End-user, 2017–2031
9.3.1. Hospitals
9.3.2. Emergency Medical Services
9.3.3. Military
9.4. Market Value Forecast, by Country, 2017–2031
9.4.1. U.S.
9.4.2. Canada
9.5. Market Attractiveness Analysis
9.5.1. By Type
9.5.2. By End-user
9.5.3. By Country
10. Europe Intraosseous Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Type, 2017–2031
10.2.1. Manual
10.2.2. Semi-automatic
10.2.3. Automatic
10.3. Market Value Forecast, by End-user, 2017–2031
10.3.1. Hospitals
10.3.2. Emergency Medical Services
10.3.3. Military
10.4. Market Value Forecast, by Country/Sub-region, 2017–2031
10.4.1. Germany
10.4.2. U.K.
10.4.3. France
10.4.4. Italy
10.4.5. Spain
10.4.6. Rest of Europe
10.5. Market Attractiveness Analysis
10.5.1. By Type
10.5.2. By End-user
10.5.3. By Country/Sub-region
11. Asia Pacific Intraosseous Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Type, 2017–2031
11.2.1. Manual
11.2.2. Semi-automatic
11.2.3. Automatic
11.3. Market Value Forecast, by End-user, 2017–2031
11.3.1. Hospitals
11.3.2. Emergency Medical Services
11.3.3. Military
11.4. Market Value Forecast, by Country/Sub-region, 2017–2031
11.4.1. China
11.4.2. India
11.4.3. Japan
11.4.4. Australia & New Zealand
11.4.5. Rest of Asia Pacific
11.5. Market Attractiveness Analysis
11.5.1. By Type
11.5.2. By End-user
11.5.3. By Country/Sub-region
12. Latin America Intraosseous Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Type, 2017–2031
12.2.1. Manual
12.2.2. Semi-automatic
12.2.3. Automatic
12.3. Market Value Forecast, by End-user, 2017–2031
12.3.1. Hospitals
12.3.2. Emergency Medical Services
12.3.3. Military
12.4. Market Value Forecast, by Country/Sub-region, 2017–2031
12.4.1. Brazil
12.4.2. Mexico
12.4.3. Rest of Latin America
12.5. Market Attractiveness Analysis
12.5.1. By Type
12.5.2. By End-user
12.5.3. By Country/Sub-region
13. Middle East & Africa Intraosseous Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Type, 2017–2031
13.2.1. Manual
13.2.2. Semi-automatic
13.2.3. Automatic
13.3. Market Value Forecast, by End-user, 2017–2031
13.3.1. Hospitals
13.3.2. Emergency Medical Services
13.3.3. Military
13.4. Market Value Forecast, by Country/Sub-region, 2017–2031
13.4.1. GCC
13.4.2. South Africa
13.4.3. Rest of Middle East & Africa
13.5. Market Attractiveness Analysis
13.5.1. By Type
13.5.2. By End-user
13.5.3. By Country/Sub-region
14. Competition Landscape
14.1. Market Player - Competition Matrix (by tier and size of companies)
14.2. Market Share Analysis, by Company, 2021
14.3. Company Profiles
14.3.1. Aero Healthcare AU Pty Ltd.
14.3.1.1. Company Overview
14.3.1.2. Product Portfolio
14.3.1.3. SWOT Analysis
14.3.1.4. Financial Overview
14.3.1.5. Strategic Overview
14.3.2. BD
14.3.2.1. Company Overview
14.3.2.2. Product Portfolio
14.3.2.3. SWOT Analysis
14.3.2.4. Financial Overview
14.3.2.5. Strategic Overview
14.3.3. Biopsybell
14.3.3.1. Company Overview
14.3.3.2. Product Portfolio
14.3.3.3. SWOT Analysis
14.3.3.4. Financial Overview
14.3.3.5. Strategic Overview
14.3.4. Cook
14.3.4.1. Company Overview
14.3.4.2. Product Portfolio
14.3.4.3. SWOT Analysis
14.3.4.4. Financial Overview
14.3.4.5. Strategic Overview
14.3.5. Teleflex Incorporated
14.3.5.1. Company Overview
14.3.5.2. Product Portfolio
14.3.5.3. SWOT Analysis
14.3.5.4. Financial Overview
14.3.5.5. Strategic Overview
14.3.6. PAVmed, Inc.
14.3.6.1. Company Overview
14.3.6.2. Product Portfolio
14.3.6.3. SWOT Analysis
14.3.6.4. Financial Overview
14.3.6.5. Strategic Overview
14.3.7. PERSYS MEDICAL
14.3.7.1. Company Overview
14.3.7.2. Product Portfolio
14.3.7.3. SWOT Analysis
14.3.7.4. Financial Overview
14.3.7.5. Strategic Overview
List of Tables
Table 01: Global Intraosseous Devices Market Size Value (US$ Mn) Forecast, by Type 2017-2031
Table 02: Global Intraosseous Devices Market Size Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 03: Global Intraosseous Devices Market Size Value (US$ Mn) Forecast, by Region, 2017-2031
Table 04: North America Intraosseous Devices Market Size Value (US$ Mn) Forecast, by Country, 2017-2031
Table 05: North America Intraosseous Devices Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 06: Europe Intraosseous Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 07: Europe Intraosseous Devices Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 08: Europe Intraosseous Devices Market Value (US$ Mn) Forecast, by End-user, 2015–2025
Table 09: Asia Pacific Intraosseous Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 10: Asia Pacific Intraosseous Devices Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 11: Asia Pacific Intraosseous Devices Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 12: Latin America Intraosseous Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 13: Latin America Intraosseous Devices Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 14: Latin America Intraosseous Devices Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 15: Middle East & Africa Intraosseous Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 16: Middle East & Africa Intraosseous Devices Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 17: Middle East & Africa Intraosseous Devices Market Value (US$ Mn) Forecast, by End-user, 2017-2031
List of Figures
Figure 01: Global Intraosseous Devices Market Snapshot
Figure 02: Global Intraosseous Devices Market Size (US$ Mn) Forecast, 2017–2031
Figure 03: Global Intraosseous Devices Market Value Share, by Type (2021)
Figure 04: Global Intraosseous Devices Market Value Share, by End-user (2021)
Figure 05: Global Intraosseous Devices Market Value Share, by Region (2021)
Figure 06: Global Intraosseous Devices Market Value Share (%), by Type, 2017 and 2031
Figure 07: Global Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Manual, 2017–2031
Figure 08: Global Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Semi-automatic, 2017–2031
Figure 09: Global Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Automatic, 2017–2031
Figure 10: Global Intraosseous Devices Market Attractiveness Analysis, by Type, 2022–2031
Figure 11: Global Intraosseous Devices Market Value Share (%), by End-user, 2017 and 2031
Figure 12: Global Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Hospitals, 2017–2031
Figure 13: Global Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Emergency Medical Services, 2017–2031
Figure 14: Global Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, by Military, 2017–2031
Figure 15: Global Intraosseous Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 16: Global Intraosseous Devices Market Scenario, 2021–2031
Figure 17: Global Intraosseous Devices Market Value Share (%), by Region, 2017 and 2031
Figure 18: Global Intraosseous Devices Market Attractiveness Analysis, by Region, 2022–2031
Figure 19: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 20: North America Intraosseous Devices Market Attractiveness Analysis, by Country, 2022–2031
Figure 21: North America Intraosseous Devices Market Value Share (%), by Country, 2017 and 2031
Figure 22: North America Intraosseous Devices Market Value (%) Share, by Type, 2017 and 2031
Figure 23: North America Intraosseous Devices Market Attractiveness Analysis, by Type, 2022–2031
Figure 24: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Manual, 2017–2031
Figure 25: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Semi-automatic, 2017–2031
Figure 26: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Automatic, 2017–2031
Figure 27: North America Intraosseous Devices Market Value Share (%), by End-user, 2017 and 2031
Figure 28: North America Market Attractiveness Analysis, by End-user, 2022–2031
Figure 29: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Hospitals, 2021–2031
Figure 30: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Emergency Medical Services (EMS), 2017–2031
Figure 31: North America Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Military, 2017–2031
Figure 32: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 33: Europe Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 34: Europe Intraosseous Devices Market Value Share (%), by Country/Sub-region, 2017 and 2031
Figure 35: Europe Intraosseous Devices Market Value Share (%), by Type, 2017 and 2031
Figure 36: Europe Intraosseous Devices Market Value Share (%), by End-user, 2017 and 2031
Figure 37: Europe Intraosseous Devices Market Attractiveness Analysis, by Type, 2022–2031
Figure 38: Europe Intraosseous Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 39: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Manual, 2017–2031
Figure 40: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Semi-automatic, 2017–2031
Figure 41: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Automatic, 2017–2031
Figure 42: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Hospitals, 2017–2031
Figure 43: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Emergency Medical Services (EMS), 207–2031
Figure 44: Europe Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Military, 2017–2031
Figure 45: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 46: Asia Pacific Intraosseous Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 47: Asia Pacific Intraosseous Devices Market Value Share (%), by Country/Sub-region, 2017 and 2031
Figure 48: Asia Pacific Intraosseous Devices Market Value Share (%), by Type, 2017 and 2031
Figure 59: Asia Pacific Intraosseous Devices Market Value Share (%), by End-user, 2017 and 2031
Figure 50: Asia Pacific Intraosseous Devices Market Attractiveness Analysis, by Type, 2022–2031
Figure 51: Asia Pacific Intraosseous Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 52: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Manual, 2017–2031
Figure 53: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Semi-automatic, 2017–2031
Figure 54: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Automatic, 2017–2031
Figure 55: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Hospitals, 2017–2031
Figure 56: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Emergency Medical Services (EMS), 2017–2031
Figure 57: Asia Pacific Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Military, 2017–2031
Figure 58: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 59: Latin America Intraosseous Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 60: Latin America Intraosseous Devices Market Value Share (%), by Country/Sub-region, 2017 and 2031
Figure 61: Latin America Intraosseous Devices Market Value Share, by Type, 2017 and 2031
Figure 62: Latin America Intraosseous Devices Market Value Share (%), by End-user, 2017 and 2031
Figure 63: Latin America Intraosseous Devices Market Attractiveness Analysis, by Type, 2022–2031
Figure 64: Latin America Intraosseous Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 65: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Manual, 2017–2031
Figure 66: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Semi-automatic, 2017–2031
Figure 67: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Automatic, 2017–2031
Figure 68: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Hospitals, 2017–2031
Figure 69: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Emergency Medical Services (EMS), 2017–2031
Figure 70: Latin America Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Military, 2017–2031
Figure 71: Middle East & Africa Intraosseous Devices Market Size (US$ Mn) Forecast, 2017–2031
Figure 72: Middle East & Africa Intraosseous Devices Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 73: Middle East & Africa Intraosseous Market Value Share (%), by Country/Sub-region, 2017 and 2031
Figure 74: Middle East & Africa Intraosseous Devices Market Value Share (%), by Type, 2017 and 2031
Figure 75: Middle East & Africa Intraosseous Devices Market Value Share (%), by End-user, 2017 and 2031
Figure 76: Middle East & Africa Intraosseous Devices Market Attractiveness Analysis, by Type, 2022–2031
Figure 77: Middle East & Africa Intraosseous Devices Market Attractiveness Analysis, by End-user, 2022–2031
Figure 78: Middle East & Africa Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Manual, 2017–2031
Figure 79: Middle East & Africa Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Semi-automatic, 2017–2031
Figure 80: Middle East & Africa Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Automatic, 2017–2031
Figure 81: Middle East & Africa Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Hospitals, 2017–2031
Figure 82: Middle East & Africa Intraosseous Devices Market Revenue (US$ Mn) Forecast, by Emergency Medical Services (EMS), 2017–2031
Figure 83: Middle East & Africa Intraosseous Devices Market Revenue (US$ Mn) Forecast and by Military, 2017–2031