Reports
Reports
The drug-device combinations market is driven by the increasing incidences of chronic diseases such as diabetes, cardiovascular issues, and cancer, which contribute to demand for combination therapeutic solutions. In addition, technological advancements such as the emergence of smart drug delivery systems and wearable devices will contribute to better treatment results and increased patient adherence.
However, the markets for drug-device combinations face barriers such as regulatory requirements and high costs that increase the burden, and can often delay products from launching or limit entry into the market entirely for smaller companies.
.webp)
There are opportunities from supplementing targeted therapies with combination therapies, consumer-driven demand for personalized medicines, expanding biotechnology advancements, and the growth of self-administered drug delivery systems that limit healthcare burden and increase patient convenience
A drug-device combination product that consists of a drug and a device, or a biological product and a device, or a drug, device, and a biological product, where the drug or biological product is packaged with the device or included in the device. The fundamental aspect of a drug-device combination product is that it incorporates either a drug or biologic with a device with the aim of achieving a defined therapeutic or diagnostic effect.
| Attribute | Detail |
|---|---|
| Market Drivers |
|
The rise of chronic diseases drives the market for drug-device combination products. Chronic diseases such as cardiovascular diseases, diabetes, and respiratory diseases necessitate long-term management. This is often achieved with both - pharmaceutical products and medical devices.
Drug-device combination products include products like insulin pumps and drug-eluting stents that are used together and can combine the capabilities of both - the drug and device to create a more effective treatment series while also requiring less adherence by the patient. For example, insulin pumps combined with continuous glucose monitor systems will facilitate accurate insulin delivery and provide real-time data on the patient's blood glucose, which supports better blood sugar control by the patient.
Chronic diseases are becoming more frequent worldwide, making new treatment innovative solutions even more important. The increasing burden of chronic diseases creates demand in the drug-device combination products space.
Advancements in technology are driving the drug-device combination products market by facilitating smarter, better, and more patient-centered therapeutic solutions. Products like smart inhalers, transdermal patches with microneedles, wearable injectors, connected Bluetooth drug delivery pens, and digital pills change the way medications are delivered and often captured in real-time or on behalf of the patient.
These advancements improve treatment and patient outcomes by providing more precise treatment control through management of dosing, data collection, and monitoring, which promotes patient adherence and engagement. The presence of IoT-connectivity, AI-based analytics, and EHR solutions enable interoperability between devices and infrastructures for value-based care and personalized medicine.
Injectable drug delivery devices are the leading force powering growth of the drug‑device combination products market. Their capability to deliver complex biologics and specialized targeted therapies that are not suitable for oral delivery has increased uptake of drug-device combination products, particularly user-friendly injectables that include autoinjectors, prefilled syringes, and pen injectors.
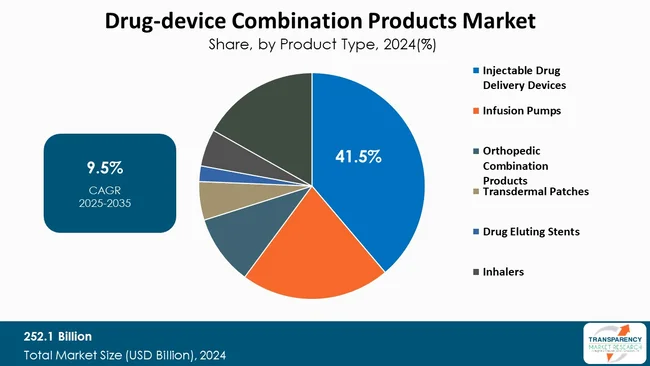
As patients seek convenience, the growing acceptance of self-administerable injectables is supported by designs that place patience comfort and adherence at the top of the list and with the move toward homecare and increasing patient self-management. In addition, the injectable drug delivery device area has the benefit of being able to accelerate innovations that are driven by technological advances such as wearable injectors, smart-delivery devices that are digitally connected, and innovative formulation products that provide accuracy in dosing and shelf life stability.
| Attribute | Detail |
|---|---|
| Leading Region | North America |
North America is at the forefront of the drug-device combination products market as a result of several factors. These include the high prevalence of chronic conditions in the region (such as diabetes, cardiovascular diseases, and cancer), a robust healthcare system, a large amount of healthcare expenditures, and encouraging reimbursement policies and public support as well as major players in the industry and ongoing improvement in medical technology.
Having a positive regulatory environment and agencies such as the U.S. Food & Drug Administration (FDA) that creates clear definitions and guidelines for drug-device combination products leads to expedited approval processes in North America, creating the framework to develop and market drug-device combination products. All of these factors illustrate the significant advantages that North America has in the drug-device combination products market segment.
Abbott, Eli Lilly and Company, Medtronic, Novo Nordisk A/S, Novartis AG, Sanofi, Boston Scientific Corporation and its affiliates, BD, Merck KGaA, B. Braun SE, Stryker, Gerresheimer AG, Narang Medical Limited., and Sparsha Pharma International Pvt. Ltd. are some of the leading players operating in the global drug-device combination products market.
Each of these players has been profiled in the drug-device combination products market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2024 | US$ 252.1 Bn |
| Forecast Value in 2035 | US$ 684.6 Bn |
| CAGR | 9.5% |
| Forecast Period | 2025-2035 |
| Historical Data Available for | 2020-2023 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 252.1 Bn in 2024.
It is projected to reach more than US$ 684.6 Bn by the end of 2035.
Increasing prevalence of chronic diseases and technological advancements.
The CAGR is anticipated to be 9.5% from 2025 to 2035.
North America is expected to account for the largest share from 2025 to 2035.
Abbott, Eli Lilly and Company, Medtronic, Novo Nordisk A/S, Novartis AG, Sanofi, Boston Scientific Corporation or its affiliates., BD, Merck KGaA, B. Braun SE , Stryker, Gerresheimer AG, Narang Medical Limited., Sparsha Pharma International Pvt. Ltd., and the other prominent players.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary : Global Drug-device Combination Products Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Drug-device Combination Products Market Analysis and Forecasts, 2020 to 2035
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Regulatory Landscape across Key Regions / Countries
5.2. Market Trends
5.3. PORTER’s Five Forces Analysis
5.4. PESTEL Analysis
5.5. Key Purchase Metrics for End-users
5.6. Brand and Pricing Analysis
5.7. Value Chain Analysis
5.8. Technological Advancement
6. Global Drug-device Combination Products Market Analysis and Forecasts, By Product Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast By Product Type, 2020 to 2035
6.3.1. Injectable Drug Delivery Devices
6.3.1.1. Pen Injectors
6.3.1.2. Autoinjectors
6.3.1.3. Pre-Filled Syringes
6.3.1.4. Others
6.3.2. Infusion Pumps
6.3.2.1. Volumetric
6.3.2.2. Syringes
6.3.2.3. Ambulatory
6.3.2.4. Implantable
6.3.2.5. Insulin
6.3.2.6. Others
6.3.3. Orthopedic Combination Products
6.3.3.1. Bone Graft Implants
6.3.3.2. Antibiotic Bone Cement
6.3.4. Transdermal Patches
6.3.5. Drug Eluting Stents
6.3.5.1. Peripheral Vascular Stents
6.3.5.2. Drug Eluting Stents
6.3.6. Inhalers
6.3.6.1. Dry Powder
6.3.6.2. Nebulizers
6.3.6.3. Metered Dose
6.3.7. Others
6.4. Market Attractiveness By Product Type
7. Global Drug-device Combination Products Market Analysis and Forecasts, By Application
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast By Application, 2020 to 2035
7.3.1. Diabetes Management
7.3.2. Respiratory Diseases
7.3.3. Eye Diseases
7.3.4. Autoimmune Diseases
7.3.5. Cancer
7.3.6. Pain Management
7.3.7. Infectious Diseases
7.3.8. Cardiovascular Diseases
7.3.9. Obesity Management
7.3.10. Others
7.4. Market Attractiveness By Application
8. Global Drug-device Combination Products Market Analysis and Forecasts, By End-user
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast By End-user, 2020 to 2035
8.3.1. Hospitals
8.3.2. Clinics
8.3.3. Ambulatory Surgical Centers
8.3.4. Home Care Centers
8.3.5. Others
8.4. Market Attractiveness By End-user
9. Global Drug-device Combination Products Market Analysis and Forecasts, By Region
9.1. Key Findings
9.2. Market Value Forecast By Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness By Region
10. North America Drug-device Combination Products Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast By Product Type, 2020 to 2035
10.2.1. Injectable Drug Delivery Devices
10.2.1.1. Pen Injectors
10.2.1.2. Autoinjectors
10.2.1.3. Pre-Filled Syringes
10.2.1.4. Others
10.2.2. Infusion Pumps
10.2.2.1. Volumetric
10.2.2.2. Syringes
10.2.2.3. Ambulatory
10.2.2.4. Implantable
10.2.2.5. Insulin
10.2.2.6. Others
10.2.3. Orthopedic Combination Products
10.2.3.1. Bone Graft Implants
10.2.3.2. Antibiotic Bone Cement
10.2.4. Transdermal Patches
10.2.5. Drug Eluting Stents
10.2.5.1. Peripheral Vascular Stents
10.2.5.2. Drug Eluting Stents
10.2.6. Inhalers
10.2.6.1. Dry Powder
10.2.6.2. Nebulizers
10.2.6.3. Metered Dose
10.2.7. Others
10.3. Market Value Forecast By Application, 2020 to 2035
10.3.1. Diabetes Management
10.3.2. Respiratory Diseases
10.3.3. Eye Diseases
10.3.4. Autoimmune Diseases
10.3.5. Cancer
10.3.6. Pain Management
10.3.7. Infectious Diseases
10.3.8. Cardiovascular Diseases
10.3.9. Obesity Management
10.3.10. Others
10.4. Market Value Forecast By End-user, 2020 to 2035
10.4.1. Hospitals
10.4.2. Clinics
10.4.3. Ambulatory Surgical Centers
10.4.4. Home Care Centers
10.4.5. Others
10.5. Market Value Forecast By Country, 2020 to 2035
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Product Type
10.6.2. By Application
10.6.3. By End-user
10.6.4. By Country
11. Europe Drug-device Combination Products Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast By Product Type, 2020 to 2035
11.2.1. Injectable Drug Delivery Devices
11.2.1.1. Pen Injectors
11.2.1.2. Autoinjectors
11.2.1.3. Pre-Filled Syringes
11.2.1.4. Others
11.2.2. Infusion Pumps
11.2.2.1. Volumetric
11.2.2.2. Syringes
11.2.2.3. Ambulatory
11.2.2.4. Implantable
11.2.2.5. Insulin
11.2.2.6. Others
11.2.3. Orthopedic Combination Products
11.2.3.1. Bone Graft Implants
11.2.3.2. Antibiotic Bone Cement
11.2.4. Transdermal Patches
11.2.5. Drug Eluting Stents
11.2.5.1. Peripheral Vascular Stents
11.2.5.2. Drug Eluting Stents
11.2.6. Inhalers
11.2.6.1. Dry Powder
11.2.6.2. Nebulizers
11.2.6.3. Metered Dose
11.2.7. Others
11.3. Market Value Forecast By Application, 2020 to 2035
11.3.1. Diabetes Management
11.3.2. Respiratory Diseases
11.3.3. Eye Diseases
11.3.4. Autoimmune Diseases
11.3.5. Cancer
11.3.6. Pain Management
11.3.7. Infectious Diseases
11.3.8. Cardiovascular Diseases
11.3.9. Obesity Management
11.3.10. Others
11.4. Market Value Forecast By End-user, 2020 to 2035
11.4.1. Hospitals
11.4.2. Clinics
11.4.3. Ambulatory Surgical Centers
11.4.4. Home Care Centers
11.4.5. Others
11.5. Market Value Forecast By Country/Sub-region, 2020 to 2035
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Italy
11.5.5. Spain
11.5.6. Switzerland
11.5.7. The Netherlands
11.5.8. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Product Type
11.6.2. By Application
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Drug-device Combination Products Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast By Product Type, 2020 to 2035
12.2.1. Injectable Drug Delivery Devices
12.2.1.1. Pen Injectors
12.2.1.2. Autoinjectors
12.2.1.3. Pre-Filled Syringes
12.2.1.4. Others
12.2.2. Infusion Pumps
12.2.2.1. Volumetric
12.2.2.2. Syringes
12.2.2.3. Ambulatory
12.2.2.4. Implantable
12.2.2.5. Insulin
12.2.2.6. Others
12.2.3. Orthopedic Combination Products
12.2.3.1. Bone Graft Implants
12.2.3.2. Antibiotic Bone Cement
12.2.4. Transdermal Patches
12.2.5. Drug Eluting Stents
12.2.5.1. Peripheral Vascular Stents
12.2.5.2. Drug Eluting Stents
12.2.6. Inhalers
12.2.6.1. Dry Powder
12.2.6.2. Nebulizers
12.2.6.3. Metered Dose
12.2.7. Others
12.3. Market Value Forecast By Application, 2020 to 2035
12.3.1. Diabetes Management
12.3.2. Respiratory Diseases
12.3.3. Eye Diseases
12.3.4. Autoimmune Diseases
12.3.5. Cancer
12.3.6. Pain Management
12.3.7. Infectious Diseases
12.3.8. Cardiovascular Diseases
12.3.9. Obesity Management
12.3.10. Others
12.4. Market Value Forecast By End-user, 2020 to 2035
12.4.1. Hospitals
12.4.2. Clinics
12.4.3. Ambulatory Surgical Centers
12.4.4. Home Care Centers
12.4.5. Others
12.5. Market Value Forecast By Country/Sub-region, 2020 to 2035
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. South Korea
12.5.6. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Product Type
12.6.2. By Application
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America Drug-device Combination Products Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast By Product Type, 2020 to 2035
13.2.1. Injectable Drug Delivery Devices
13.2.1.1. Pen Injectors
13.2.1.2. Autoinjectors
13.2.1.3. Pre-Filled Syringes
13.2.1.4. Others
13.2.2. Infusion Pumps
13.2.2.1. Volumetric
13.2.2.2. Syringes
13.2.2.3. Ambulatory
13.2.2.4. Implantable
13.2.2.5. Insulin
13.2.2.6. Others
13.2.3. Orthopedic Combination Products
13.2.3.1. Bone Graft Implants
13.2.3.2. Antibiotic Bone Cement
13.2.4. Transdermal Patches
13.2.5. Drug Eluting Stents
13.2.5.1. Peripheral Vascular Stents
13.2.5.2. Drug Eluting Stents
13.2.6. Inhalers
13.2.6.1. Dry Powder
13.2.6.2. Nebulizers
13.2.6.3. Metered Dose
13.2.7. Others
13.3. Market Value Forecast By Application, 2020 to 2035
13.3.1. Diabetes Management
13.3.2. Respiratory Diseases
13.3.3. Eye Diseases
13.3.4. Autoimmune Diseases
13.3.5. Cancer
13.3.6. Pain Management
13.3.7. Infectious Diseases
13.3.8. Cardiovascular Diseases
13.3.9. Obesity Management
13.3.10. Others
13.4. Market Value Forecast By End-user, 2020 to 2035
13.4.1. Hospitals
13.4.2. Clinics
13.4.3. Ambulatory Surgical Centers
13.4.4. Home Care Centers
13.4.5. Others
13.5. Market Value Forecast By Country/Sub-region, 2020 to 2035
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Argentina
13.5.4. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Product Type
13.6.2. By Application
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Drug-device Combination Products Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast By Product Type, 2020 to 2035
14.2.1. Injectable Drug Delivery Devices
14.2.1.1. Pen Injectors
14.2.1.2. Autoinjectors
14.2.1.3. Pre-Filled Syringes
14.2.1.4. Others
14.2.2. Infusion Pumps
14.2.2.1. Volumetric
14.2.2.2. Syringes
14.2.2.3. Ambulatory
14.2.2.4. Implantable
14.2.2.5. Insulin
14.2.2.6. Others
14.2.3. Orthopedic Combination Products
14.2.3.1. Bone Graft Implants
14.2.3.2. Antibiotic Bone Cement
14.2.4. Transdermal Patches
14.2.5. Drug Eluting Stents
14.2.5.1. Peripheral Vascular Stents
14.2.5.2. Drug Eluting Stents
14.2.6. Inhalers
14.2.6.1. Dry Powder
14.2.6.2. Nebulizers
14.2.6.3. Metered Dose
14.2.7. Others
14.3. Market Value Forecast By Application, 2020 to 2035
14.3.1. Diabetes Management
14.3.2. Respiratory Diseases
14.3.3. Eye Diseases
14.3.4. Autoimmune Diseases
14.3.5. Cancer
14.3.6. Pain Management
14.3.7. Infectious Diseases
14.3.8. Cardiovascular Diseases
14.3.9. Obesity Management
14.3.10. Others
14.4. Market Value Forecast By End-user, 2020 to 2035
14.4.1. Hospitals
14.4.2. Clinics
14.4.3. Ambulatory Surgical Centers
14.4.4. Home Care Centers
14.4.5. Others
14.5. Market Value Forecast By Country/Sub-region, 2020 to 2035
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Product Type
14.6.2. By Application
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player – Competition Matrix (By Tier and Size of companies)
15.2. Market Share Analysis By Company (2024)
15.3. Company Profiles
15.3.1. Abbott
15.3.1.1. Company Overview
15.3.1.2. Financial Overview
15.3.1.3. Product Portfolio
15.3.1.4. Business Strategies
15.3.1.5. Recent Developments
15.3.2. Eli Lilly and Company
15.3.2.1. Company Overview
15.3.2.2. Financial Overview
15.3.2.3. Product Portfolio
15.3.2.4. Business Strategies
15.3.2.5. Recent Developments
15.3.3. Medtronic
15.3.3.1. Company Overview
15.3.3.2. Financial Overview
15.3.3.3. Product Portfolio
15.3.3.4. Business Strategies
15.3.3.5. Recent Developments
15.3.4. Novo Nordisk A/S
15.3.4.1. Company Overview
15.3.4.2. Financial Overview
15.3.4.3. Product Portfolio
15.3.4.4. Business Strategies
15.3.4.5. Recent Developments
15.3.5. Novartis AG
15.3.5.1. Company Overview
15.3.5.2. Financial Overview
15.3.5.3. Product Portfolio
15.3.5.4. Business Strategies
15.3.5.5. Recent Developments
15.3.6. Sanofi
15.3.6.1. Company Overview
15.3.6.2. Financial Overview
15.3.6.3. Product Portfolio
15.3.6.4. Business Strategies
15.3.6.5. Recent Developments
15.3.7. Boston Scientific Corporation or its affiliates.
15.3.7.1. Company Overview
15.3.7.2. Financial Overview
15.3.7.3. Product Portfolio
15.3.7.4. Business Strategies
15.3.7.5. Recent Developments
15.3.8. BD
15.3.8.1. Company Overview
15.3.8.2. Financial Overview
15.3.8.3. Product Portfolio
15.3.8.4. Business Strategies
15.3.8.5. Recent Developments
15.3.9. Merck KGaA
15.3.9.1. Company Overview
15.3.9.2. Financial Overview
15.3.9.3. Product Portfolio
15.3.9.4. Business Strategies
15.3.9.5. Recent Developments
15.3.10. B. Braun SE
15.3.10.1. Company Overview
15.3.10.2. Financial Overview
15.3.10.3. Product Portfolio
15.3.10.4. Business Strategies
15.3.10.5. Recent Developments
15.3.11. Stryker
15.3.11.1. Company Overview
15.3.11.2. Financial Overview
15.3.11.3. Product Portfolio
15.3.11.4. Business Strategies
15.3.11.5. Recent Developments
15.3.12. Gerresheimer AG
15.3.12.1. Company Overview
15.3.12.2. Financial Overview
15.3.12.3. Product Portfolio
15.3.12.4. Business Strategies
15.3.12.5. Recent Developments
15.3.13. Narang Medical Limited.
15.3.13.1. Company Overview
15.3.13.2. Financial Overview
15.3.13.3. Product Portfolio
15.3.13.4. Business Strategies
15.3.13.5. Recent Developments
15.3.14. Sparsha Pharma International Pvt Ltd
15.3.14.1. Company Overview
15.3.14.2. Financial Overview
15.3.14.3. Product Portfolio
15.3.14.4. Business Strategies
15.3.14.5. Recent Developments
15.3.15. Other Prominent Players
15.3.15.1. Company Overview
15.3.15.2. Financial Overview
15.3.15.3. Product Portfolio
15.3.15.4. Business Strategies
15.3.15.5. Recent Developments
List of Tables
Table 01: Global Drug-device Combination Products Market Value (US$ Mn) Forecast, By Product Type, 2020 to 2035
Table 02: Global Drug-device Combination Products Market Value (US$ Mn) Forecast, By Application, 2020 to 2035
Table 03: Global Drug-device Combination Products Market Value (US$ Mn) Forecast, By End-user, 2020 to 2035
Table 04: Global Drug-device Combination Products Market Value (US$ Mn) Forecast, By Region, 2020 to 2035
Table 05: North America Drug-device Combination Products Market Value (US$ Mn) Forecast, by Country, 2020-2035
Table 06: North America Drug-device Combination Products Market Value (US$ Mn) Forecast, By Product Type, 2020 to 2035
Table 07: North America Drug-device Combination Products Market Value (US$ Mn) Forecast, By Application, 2020 to 2035
Table 08: North America Drug-device Combination Products Market Value (US$ Mn) Forecast, By End-user, 2020 to 2035
Table 09: Europe Drug-device Combination Products Market Value (US$ Mn) Forecast, by Country / Sub-region, 2020-2035
Table 10: Europe Drug-device Combination Products Market Value (US$ Mn) Forecast, By Product Type, 2020 to 2035
Table 11: Europe Drug-device Combination Products Market Value (US$ Mn) Forecast, By Application, 2020 to 2035
Table 12: Europe Drug-device Combination Products Market Value (US$ Mn) Forecast, By End-user, 2020 to 2035
Table 13: Asia Pacific Drug-device Combination Products Market Value (US$ Mn) Forecast, by Country / Sub-region, 2020-2035
Table 14: Asia Pacific Drug-device Combination Products Market Value (US$ Mn) Forecast, By Product Type, 2020 to 2035
Table 15 Asia Pacific Drug-device Combination Products Market Value (US$ Mn) Forecast, By Application, 2020 to 2035
Table 16: Asia Pacific Drug-device Combination Products Market Value (US$ Mn) Forecast, By End-user, 2020 to 2035
Table 17: Latin America Drug-device Combination Products Market Value (US$ Mn) Forecast, by Country / Sub-region, 2020-2035
Table 18: Latin America Drug-device Combination Products Market Value (US$ Mn) Forecast, By Product Type, 2020 to 2035
Table 19: Latin America Drug-device Combination Products Market Value (US$ Mn) Forecast, By Application, 2020 to 2035
Table 20: Latin America Drug-device Combination Products Market Value (US$ Mn) Forecast, By End-user, 2020 to 2035
Table 21: Middle East & Africa Drug-device Combination Products Market Value (US$ Mn) Forecast, by Country / Sub-region, 2020-2035
Table 22: Middle East & Africa Drug-device Combination Products Market Value (US$ Mn) Forecast, By Product Type, 2020 to 2035
Table 23: Middle East & Africa Drug-device Combination Products Market Value (US$ Mn) Forecast, By Application, 2020 to 2035
Table 24: Middle East & Africa Drug-device Combination Products Market Value (US$ Mn) Forecast, By End-user, 2020 to 2035
List of Figures
Figure 01: Global Drug-device Combination Products Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 02: Global Drug-device Combination Products Market Attractiveness Analysis, By Product Type, 2025 to 2035
Figure 03: Global Drug-device Combination Products Market Revenue (US$ Mn), by Injectable Drug Delivery Devices, 2020 to 2035
Figure 04: Global Drug-device Combination Products Market Revenue (US$ Mn), by Infusion Pumps, 2020 to 2035
Figure 05: Global Drug-device Combination Products Market Revenue (US$ Mn), By Orthopedic Combination Products, 2020 to 2035
Figure 06: Global Drug-device Combination Products Market Revenue (US$ Mn), By Transdermal Patches, 2020 to 2035
Figure 07: Global Drug-device Combination Products Market Revenue (US$ Mn), By Drug Eluting Stents, 2020 to 2035
Figure 08: Global Drug-device Combination Products Market Revenue (US$ Mn), By Inhalers, 2020 to 2035
Figure 09: Global Drug-device Combination Products Market Revenue (US$ Mn), By Others, 2020 to 2035
Figure 10: Global Drug-device Combination Products Market Value Share Analysis, By Application, 2024 and 2035
Figure 11: Global Drug-device Combination Products Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 12: Global Drug-device Combination Products Market Revenue (US$ Mn), by Diabetes Management, 2020 to 2035
Figure 13: Global Drug-device Combination Products Market Revenue (US$ Mn), By Respiratory Diseases, 2020 to 2035
Figure 14: Global Drug-device Combination Products Market Revenue (US$ Mn), By Eye Diseases, 2020 to 2035
Figure 15: Global Drug-device Combination Products Market Revenue (US$ Mn), By Autoimmune Diseases, 2020 to 2035
Figure 16: Global Drug-device Combination Products Market Revenue (US$ Mn), By Cancer, 2020 to 2035
Figure 17: Global Drug-device Combination Products Market Revenue (US$ Mn), By Pain Management, 2020 to 2035
Figure 18: Global Drug-device Combination Products Market Revenue (US$ Mn), By Infectious Diseases, 2020 to 2035
Figure 19: Global Drug-device Combination Products Market Revenue (US$ Mn), By Cardiovascular Diseases, 2020 to 2035
Figure 20: Global Drug-device Combination Products Market Revenue (US$ Mn), By Obesity Management, 2020 to 2035
Figure 21: Global Drug-device Combination Products Market Revenue (US$ Mn), By Others, 2020 to 2035
Figure 22: Global Drug-device Combination Products Market Value Share Analysis, By End-user, 2024 and 2035
Figure 23: Global Drug-device Combination Products Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 24: Global Drug-device Combination Products Market Revenue (US$ Mn), by Hospitals, 2020 to 2035
Figure 25: Global Drug-device Combination Products Market Revenue (US$ Mn), By Clinics, 2020 to 2035
Figure 26: Global Drug-device Combination Products Market Revenue (US$ Mn), By Ambulatory Surgical Centers, 2020 to 2035
Figure 27: Global Drug-device Combination Products Market Revenue (US$ Mn), By Home Care Centers, 2020 to 2035
Figure 28: Global Drug-device Combination Products Market Revenue (US$ Mn), By Others, 2020 to 2035
Figure 29: Global Drug-device Combination Products Market Value Share Analysis, By Region, 2024 and 2035
Figure 30: Global Drug-device Combination Products Market Attractiveness Analysis, By Region, 2025 to 2035
Figure 31: North America Drug-device Combination Products Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 32: North America Drug-device Combination Products Market Value Share Analysis, by Country, 2024 and 2035
Figure 33: North America Drug-device Combination Products Market Attractiveness Analysis, by Country, 2025 to 2035
Figure 34: North America Drug-device Combination Products Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 35: North America Drug-device Combination Products Market Attractiveness Analysis, By Product Type, 2025 to 2035
Figure 36: North America Drug-device Combination Products Market Value Share Analysis, By Application, 2024 and 2035
Figure 37: North America Drug-device Combination Products Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 38: North America Drug-device Combination Products Market Value Share Analysis, By End-user, 2024 and 2035
Figure 39: North America Drug-device Combination Products Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 40: Europe Drug-device Combination Products Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 41: Europe Drug-device Combination Products Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 42: Europe Drug-device Combination Products Market Attractiveness Analysis, by Country / Sub-region, 2025 to 2035
Figure 43: Europe Drug-device Combination Products Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 44: Europe Drug-device Combination Products Market Attractiveness Analysis, By Product Type, 2025 to 2035
Figure 45: Europe Drug-device Combination Products Market Value Share Analysis, By Application, 2024 and 2035
Figure 46: Europe Drug-device Combination Products Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 47: Europe Drug-device Combination Products Market Value Share Analysis, By End-user, 2024 and 2035
Figure 48: Europe Drug-device Combination Products Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 49: Asia Pacific Drug-device Combination Products Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 50: Asia Pacific Drug-device Combination Products Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 51: Asia Pacific Drug-device Combination Products Market Attractiveness Analysis, by Country / Sub-region, 2025 to 2035
Figure 52: Asia Pacific Drug-device Combination Products Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 53: Asia Pacific Drug-device Combination Products Market Attractiveness Analysis, By Product Type, 2025 to 2035
Figure 54: Asia Pacific Drug-device Combination Products Market Value Share Analysis, By Application, 2024 and 2035
Figure 55: Asia Pacific Drug-device Combination Products Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 56: Asia Pacific Drug-device Combination Products Market Value Share Analysis, By End-user, 2024 and 2035
Figure 57: Asia Pacific Drug-device Combination Products Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 58: Latin America Drug-device Combination Products Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 59: Latin America Drug-device Combination Products Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 60: Latin America Drug-device Combination Products Market Attractiveness Analysis, by Country / Sub-region, 2025 to 2035
Figure 61: Latin America Drug-device Combination Products Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 62: Latin America Drug-device Combination Products Market Attractiveness Analysis, By Product Type, 2025 to 2035
Figure 63: Latin America Drug-device Combination Products Market Value Share Analysis, By Application, 2024 and 2035
Figure 64: Latin America Drug-device Combination Products Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 65: Latin America Drug-device Combination Products Market Value Share Analysis, By End-user, 2024 and 2035
Figure 66: Latin America Drug-device Combination Products Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 67: Middle East & Africa Drug-device Combination Products Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 68: Middle East & Africa Drug-device Combination Products Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 69: Middle East & Africa Drug-device Combination Products Market Attractiveness Analysis, by Country / Sub-region, 2025 to 2035
Figure 70: Middle East & Africa Drug-device Combination Products Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 71: Middle East & Africa Drug-device Combination Products Market Attractiveness Analysis, By Product Type, 2025 to 2035
Figure 72: Middle East & Africa Drug-device Combination Products Market Value Share Analysis, By Application, 2024 and 2035
Figure 73: Middle East & Africa Drug-device Combination Products Market Attractiveness Analysis, By Application, 2025 to 2035
Figure 74: Middle East & Africa Drug-device Combination Products Market Value Share Analysis, By End-user, 2024 and 2035
Figure 75: Middle East & Africa Drug-device Combination Products Market Attractiveness Analysis, By End-user, 2025 to 2035