Reports
Reports
Analysts’ Viewpoint on Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Scenario
Exposure to tobacco smoke, which includes secondhand or passive exposure, allergens (such as pollen, ragweed, dust mites, or animal dander), irritants in the air (such as chemical fumes, smoke, or strong odors), and extreme weather conditions are the major risk factors driving the global chronic obstructive pulmonary disease treatment market. Other risk factors such as indoor air pollution (such as solid fuel used for heating and cooking), outdoor air pollution, occupational dust and chemicals (such as irritants, vapors, and fumes), and frequent lower respiratory infections during childhood are also propelling the global chronic obstructive pulmonary disease treatment market. Key companies operating in the market are focusing on developing low-cost and effective drugs for the treatment of chronic obstructive pulmonary diseases to increase their global footprint.
Chronic obstructive pulmonary disease is a group of conditions affecting the lungs that lead to difficulty in breathing. Chronic bronchitis and emphysema are the two major types of chronic obstructive pulmonary diseases. Breathlessness is a key characteristic of COPD. In case of emphysema, the alveoli (tiny air sac) in the lungs is damaged. The walls of the alveoli are stretched, making the lungs bigger and resulting in difficulty in the movement of air. In chronic bronchitis, bronchial airways (breathing tubes) inside the lungs are inflamed. High prevalence of COPD across the globe and rich pipeline of drugs for COPD treatment are likely to drive the global market during the forecast period. Furthermore, increase in geriatric population and unhealthy lifestyle choices among various age groups are projected to propel the chronic obstructive pulmonary disease (COPD) treatment market in the near future.
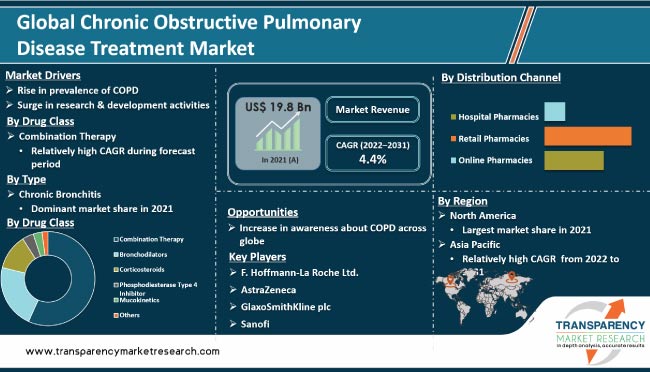
Chronic obstructive pulmonary disease (COPD) is a major health concern affecting a large number of people across the globe. The disease burden is rising, thereby increasing the need for COPD management. Tobacco smoking could lead to COPD. The number of adults addicted to smoking has been increasing across the globe. Other risk factors include occupation and indoor air pollution. According to the WHO, the primary cause of COPD is exposure to tobacco smoke (either active smoking or passive smoking). According to the organization, chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally, accounting for 3.23 million deaths in 2019. Nearly 90% of COPD deaths in those under 70 years of age occur in low- and middle-income countries (LMIC). According to a report by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published in 2022, the prevalence and burden of COPD is projected to increase in the near future due to continued exposure to COPD risk factors, rise in incidence of interstitial lung diseases, and aging population across the world. This is anticipated to augment the chronic obstructive pulmonary disease treatment market in the near future.
Combination therapies include LAMA-ICS, LABA-ICS, triple therapy, and other combination drugs. Adoption of combination therapy is increasing, as it is more effective than separate use of bronchodilators and corticosteroids. Increase in the number of patients successfully treated with combination therapy who were not showing signs of recovery with bronchodilators or corticosteroid treatments is expected to fuel the global chronic obstructive pulmonary disease treatment market. Combination therapy is more effective than monotherapy for improving symptoms and quality of life.
In terms of drug class, the global COPD treatment market has been classified into combination therapy, bronchodilators, corticosteroids, phosphodiesterase type 4 inhibitor, mucokinetics, and others. The combination therapy segment held major share of around 50% of the global market in 2021. It is likely to dominate the global market during the forecast period, owing to the increase in efficacy of combination therapy in COPD treatment. Rise in the success rate of combination therapy in COPD treatment and recent regulatory approvals have augmented the adoption of combination therapy. This is projected to drive the segment during the forecast period.
In terms of type, the global COPD treatment market has been bifurcated into chronic bronchitis and emphysema. The chronic bronchitis segment accounted for the largest chronic obstructive pulmonary disease treatment market share in 2021. Chronic bronchitis is a group of lung diseases that causes airflow blockage and breathing issues. It is widespread among smokers and rising at a rapid pace.
Based on distribution channel, the global market has been divided into hospital pharmacies, retail pharmacies, and online pharmacies. The retail pharmacies segment is anticipated to account for major share of the global chronic obstructive pulmonary disease treatment market during the forecast period. The segment is expected to grow at a rapid pace during the forecast period, owing to the increase in number of COPD therapeutics dispensed through retail pharmacies and rise in number of retail pharmacies in developing countries.
As per chronic obstructive pulmonary disease treatment market analysis, North America accounted for the largest share of around 40% of the global market in 2021. The market in the region is projected to grow at a CAGR of 4.3% from 2022 to 2031. This can be ascribed to the highly structured health care industry and availability of well-defined reimbursement policies from private and public health insurance firms. Furthermore, rise in research & development activities to provide advanced and efficient products, and presence of a large number of players are driving the chronic obstructive pulmonary disease treatment market size in North America.
Europe held the second largest share of the global market in 2021. Well-developed health care infrastructure and increase in incidence of chronic obstructive pulmonary diseases are propelling the COPD treatment market in the region.
The market in Asia Pacific is expected to grow at the fastest CAGR of 4.7% during the forecast period. The region has a relatively untapped chronic obstructive pulmonary disease treatment market compared to developed regions. Therefore, Asia Pacific offers significant opportunities to market players. High prevalence of chronic obstructive pulmonary disease, increase in geriatric population, surge in patient population, and growth of the health care industry are propelling the chronic obstructive pulmonary disease treatment market in the region.
The global chronic obstructive pulmonary disease (COPD) treatment market is consolidated, with the presence of a small number of key players. As per chronic obstructive pulmonary disease treatment market trends, leading players in the market are adopting strategies such as new product development, product launches, product approvals, agreements, partnerships, and mergers. Key players operating in the global chronic obstructive pulmonary disease (COPD) treatment market are Almirall, AstraZeneca, Boehringer Ingelheim International GmbH, CHIESI Farmaceutici S.p.A., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Kyowa Hakko Kirin, Mylan N.V., Novartis AG, Orion Corporation, Sanofi, Sunovion Pharmaceuticals, Inc. (Sumitomo Dainippon Pharma Co., Ltd.), Teva Pharmaceutical Industries Ltd., Theravance Biopharma, and Verona Pharmaceuticals.
Each of these players has been profiled in the chronic obstructive pulmonary disease (COPD) treatment market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Market Size Value in 2021 |
US$ 19.8 Bn |
|
Market Forecast Value in 2031 |
More than US$ 30.4 Bn |
|
Growth Rate |
4.4% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2020 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
|
Competition Landscape |
Market share analysis by company (2021) Company profiles section includes overview, product portfolio, sales footprint, key subsidiaries or distributors, strategy & recent developments, and key financials. |
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global chronic obstructive pulmonary disease (COPD) treatment market was valued at US$ 19.8 Bn in 2021.
The global chronic obstructive pulmonary disease (COPD) treatment market is projected to reach more than US$ 30.4 Bn by 2031.
The global chronic obstructive pulmonary disease (COPD) treatment market is anticipated to grow at a CAGR of 4.4% from 2022 to 2031.
Rise in prevalence of chronic obstructive pulmonary disease (COPD) leading to an increase in demand for testing & diagnosis, surge in research & development activities, and growth in awareness about COPD.
The combination therapy segment held around 55% share of the global market in 2021.
North America is expected to account for major share of the global chronic obstructive pulmonary disease (COPD) treatment market during the forecast period.
F. Hoffmann-La Roche Ltd., AstraZeneca, GlaxoSmithKline plc, Sanofi, Novartis AG, Teva Pharmaceutical Industries Ltd., Mylan N.V., Boehringer Ingelheim International GmbH, Sunovion Pharmaceuticals, Inc. (Sumitomo Dainippon Pharma Co., Ltd.)., CHIESI Farmaceutici S.p.A., Orion Corporation, Almirall, Theravance Biopharma, Verona Pharmaceuticals, and Kyowa Hakko Kirin.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlight
2. Assumptions and Research Methodology
3. Executive Summary: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Regulatory Scenario by Region/globally
5.2. Reimbursement Scenario by Region/globally
5.3. Pipeline Analysis
5.4. Technological Advancements
5.5. Pricing Analysis (Brand pricing, Average Selling Price by Region/Country)
5.6. Disease Prevalence & Incidence Rate globally with key countries
5.7. Key Industry Events (mergers, acquisitions, partnerships, collaborations, etc.)
5.8. COVID-19 Pandemic Impact on Industry (value chain and short / mid / long term impact)
6. Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast, by Drug Class
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Drug Class, 2017–2031
6.3.1. Combination Therapy
6.3.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
6.3.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
6.3.1.3. Triple therapy
6.3.1.4. Others
6.3.2. Bronchodilators
6.3.2.1. Long Acting Beta Agonist (LABA)
6.3.2.2. Short Acting Beta Agonist (SABA)
6.3.2.3. Long Acting Muscarinic Antagonist (LAMA)
6.3.3. Corticosteroids
6.3.4. Phosphodiesterase Type 4 Inhibitor
6.3.5. Mucokinetics
6.3.6. Others
6.4. Market Attractiveness Analysis, by Drug Class
7. Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast, by Type
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Type, 2017–2031
7.3.1. Chronic Bronchitis
7.3.2. Emphysema
7.4. Market Attractiveness Analysis, by Type
8. Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast, by Distribution Channel
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Distribution Channel, 2017–2031
8.3.1. Hospital Pharmacies
8.3.2. Retail Pharmacies
8.3.3. Online Pharmacies
8.4. Market Attractiveness Analysis, by Distribution Channel
9. Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Drug Class, 2017–2031
10.2.1. Combination Therapy
10.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
10.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
10.2.1.3. Triple therapy
10.2.1.4. Others
10.2.2. Bronchodilators
10.2.2.1. Long Acting Beta Agonist (LABA)
10.2.2.2. Short Acting Beta Agonist (SABA)
10.2.2.3. Long Acting Muscarinic Antagonist (LAMA)
10.2.3. Corticosteroids
10.2.4. Phosphodiesterase Type 4 Inhibitor
10.2.5. Mucokinetics
10.2.6. Others
10.3. Market Value Forecast, by Type, 2017–2031
10.3.1. Chronic Bronchitis
10.3.2. Emphysema
10.4. Market Value Forecast, by Distribution Channel, 2017–2031
10.4.1. Hospital Pharmacies
10.4.2. Retail Pharmacies
10.4.3. Online Pharmacies
10.5. Market Value Forecast, by Country, 2017–2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Drug Class
10.6.2. By Type
10.6.3. By Distribution Channel
10.6.4. By Country
11. Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Drug Class, 2017–2031
11.2.1. Combination Therapy
11.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
11.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
11.2.1.3. Triple therapy
11.2.1.4. Others
11.2.2. Bronchodilators
11.2.2.1. Long Acting Beta Agonist (LABA)
11.2.2.2. Short Acting Beta Agonist (SABA)
11.2.2.3. Long Acting Muscarinic Antagonist (LAMA)
11.2.3. Corticosteroids
11.2.4. Phosphodiesterase Type 4 Inhibitor
11.2.5. Mucokinetics
11.2.6. Others
11.3. Market Value Forecast, by Type, 2017–2031
11.3.1. Chronic Bronchitis
11.3.2. Emphysema
11.4. Market Value Forecast, by Distribution Channel, 2017–2031
11.4.1. Hospital Pharmacies
11.4.2. Retail Pharmacies
11.4.3. Online Pharmacies
11.5. Market Value Forecast, by Country/Sub-region, 2017–2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Drug Class
11.6.2. By Type
11.6.3. By Distribution Channel
11.6.4. By Country/Sub-region
12. Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Drug Class, 2017–2031
12.2.1. Combination Therapy
12.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
12.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
12.2.1.3. Triple therapy
12.2.1.4. Others
12.2.2. Bronchodilators
12.2.2.1. Long Acting Beta Agonist (LABA)
12.2.2.2. Short Acting Beta Agonist (SABA)
12.2.2.3. Long Acting Muscarinic Antagonist (LAMA)
12.2.3. Corticosteroids
12.2.4. Phosphodiesterase Type 4 Inhibitor
12.2.5. Mucokinetics
12.2.6. Others
12.3. Market Value Forecast, by Type, 2017–2031
12.3.1. Chronic Bronchitis
12.3.2. Emphysema
12.4. Market Value Forecast, by Distribution Channel, 2017–2031
12.4.1. Hospital Pharmacies
12.4.2. Retail Pharmacies
12.4.3. Online Pharmacies
12.5. Market Value Forecast, by Country/Sub-region, 2017–2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Drug Class
12.6.2. By Type
12.6.3. By Distribution Channel
12.6.4. By Country/Sub-region
13. Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Drug Class, 2017–2031
13.2.1. Combination Therapy
13.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
13.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
13.2.1.3. Triple therapy
13.2.1.4. Others
13.2.2. Bronchodilators
13.2.2.1. Long Acting Beta Agonist (LABA)
13.2.2.2. Short Acting Beta Agonist (SABA)
13.2.2.3. Long Acting Muscarinic Antagonist (LAMA)
13.2.3. Corticosteroids
13.2.4. Phosphodiesterase Type 4 Inhibitor
13.2.5. Mucokinetics
13.2.6. Others
13.3. Market Value Forecast, by Type, 2017–2031
13.3.1. Chronic Bronchitis
13.3.2. Emphysema
13.4. Market Value Forecast, by Distribution Channel, 2017–2031
13.4.1. Hospital Pharmacies
13.4.2. Retail Pharmacies
13.4.3. Online Pharmacies
13.5. Market Value Forecast, by Country/Sub-region, 2017–2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Drug Class
13.6.2. By Type
13.6.3. By Distribution Channel
13.6.4. By Country/Sub-region
14. Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Drug Class, 2017–2031
14.2.1. Combination Therapy
14.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
14.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
14.2.1.3. Triple therapy
14.2.1.4. Others
14.2.2. Bronchodilators
14.2.2.1. Long Acting Beta Agonist (LABA)
14.2.2.2. Short Acting Beta Agonist (SABA)
14.2.2.3. Long Acting Muscarinic Antagonist (LAMA)
14.2.3. Corticosteroids
14.2.4. Phosphodiesterase Type 4 Inhibitor
14.2.5. Mucokinetics
14.2.6. Others
14.3. Market Value Forecast, by Type, 2017–2031
14.3.1. Chronic Bronchitis
14.3.2. Emphysema
14.4. Market Value Forecast, by Distribution Channel, 2017–2031
14.4.1. Hospital Pharmacies
14.4.2. Retail Pharmacies
14.4.3. Online Pharmacies
14.5. Market Value Forecast, by Country/Sub-region, 2017–2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Drug Class
14.6.2. By Type
14.6.3. By Distribution Channel
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2021
15.3. Company Profiles
15.3.1. F. Hoffmann-La Roche Ltd.
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Financial Overview
15.3.1.4. SWOT Analysis
15.3.1.5. Strategic Overview
15.3.2. AstraZeneca
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. SWOT Analysis
15.3.2.5. Strategic Overview
15.3.3. GlaxoSmithKline plc
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Financial Overview
15.3.3.4. SWOT Analysis
15.3.3.5. Strategic Overview
15.3.4. Sanofi
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. SWOT Analysis
15.3.4.5. Strategic Overview
15.3.5. Novartis AG
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. SWOT Analysis
15.3.5.5. Strategic Overview
15.3.6. Teva Pharmaceutical Industries Ltd.
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Financial Overview
15.3.6.4. SWOT Analysis
15.3.6.5. Strategic Overview
15.3.7. Mylan N.V.
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. SWOT Analysis
15.3.7.5. Strategic Overview
15.3.8. Boehringer Ingelheim International GmbH
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Financial Overview
15.3.8.4. SWOT Analysis
15.3.8.5. Strategic Overview
15.3.9. Sunovion Pharmaceuticals Inc. (Sumitomo Dainippon Pharma Co., Ltd.)
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. Financial Overview
15.3.9.4. SWOT Analysis
15.3.9.5. Strategic Overview
15.3.10. CHIESI Farmaceutici S.p.A.
15.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.10.2. Product Portfolio
15.3.10.3. Financial Overview
15.3.10.4. SWOT Analysis
15.3.10.5. Strategic Overview
15.3.11. Orion Corporation
15.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.11.2. Product Portfolio
15.3.11.3. Financial Overview
15.3.11.4. SWOT Analysis
15.3.11.5. Strategic Overview
15.3.12. Almirall
15.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.12.2. Product Portfolio
15.3.12.3. Financial Overview
15.3.12.4. SWOT Analysis
15.3.12.5. Strategic Overview
15.3.13. Theravance Biopharma
15.3.13.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.13.2. Product Portfolio
15.3.13.3. Financial Overview
15.3.13.4. SWOT Analysis
15.3.13.5. Strategic Overview
15.3.14. Verona Pharmaceuticals
15.3.14.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.14.2. Product Portfolio
15.3.14.3. Financial Overview
15.3.14.4. SWOT Analysis
15.3.14.5. Strategic Overview
15.3.15. Kyowa Hakko Kirin
15.3.15.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.15.2. Product Portfolio
15.3.15.3. Financial Overview
15.3.15.4. SWOT Analysis
15.3.15.5. Strategic Overview
List of Tables
Table 01: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Drug Class, 2017‒2031
Table 02: Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by 2017‒2031, by Combination Therapy
Table 03: Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by 2017‒2031, by Bronchodilators
Table 04: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 05: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 06: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 07: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 08: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Drug Class, 2017‒2031
Table 09: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Combination Therapy, 2017‒2031
Table 10: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Bronchodilators, 2017‒2031
Table 11: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 12: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 13: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 14: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Drug Class, 2017‒2031
Table 15: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Combination Therapy, 2017‒2031
Table 16: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Bronchodilators, 2017‒2031
Table 17: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 18: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 19: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 20: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Drug Class, 2017‒2031
Table 21: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Combination Therapy, 2017‒2031
Table 22: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Bronchodilators, 2017‒2031
Table 23: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 24: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 25: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 26: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Drug Class, 2017‒2031
Table 27: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Combination Therapy, 2017‒2031
Table 28: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Bronchodilators, 2017‒2031
Table 29: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 30: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
Table 31: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 32: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Drug Class, 2017‒2031
Table 33: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Combination Therapy, 2017‒2031
Table 34: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Bronchodilators, 2017‒2031
Table 35: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 36: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017–2031
List of Figures
Figure 01: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share, by Drug Class, 2021
Figure 03: Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share, by Type, 2021
Figure 04: Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share, by Distribution Channel 2021
Figure 05: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2021 and 2031
Figure 06: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 07: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn), by Combination Therapy, 2017‒2031
Figure 08: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn), by Bronchodilators, 2017‒2031
Figure 09: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn), by Corticosteroids 2017‒2031
Figure 10: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn), by Phosphodiesterase Type 4 Inhibitor, 2017‒2031
Figure 11: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn), by Mucokinetics 2017‒2031
Figure 12: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn), by Others, 2017‒2031
Figure 13: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Type, 2021 and 2031
Figure 14: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Type, 2022–2031
Figure 15: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Revenue (US$ Mn), by Chronic Bronchitis, 2017–2031
Figure 16: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Revenue (US$ Mn), by Emphysema, 2017–2031
Figure 17: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2021 and 2031
Figure 18: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 19: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Revenue (US$ Mn), by Hospital Pharmacies, 2017–2031
Figure 20: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Revenue (US$ Mn), by Retail Pharmacies, 2017–2031
Figure 21: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Revenue (US$ Mn), by Online Pharmacies, 2017–2031
Figure 22: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Region, 2021 and 2031
Figure 23: Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Region, 2022–2031
Figure 24: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, 2017–2031
Figure 25: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country, 2021 and 2031
Figure 26: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country, 2022–2031
Figure 27: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2021 and 2031
Figure 28: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 29: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Type, 2021 and 2031
Figure 30: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Type, 2022–2031
Figure 31: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2021 and 2031
Figure 32: North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 33: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, 2017–2031
Figure 34: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 35: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 36: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2021 and 2031
Figure 37: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 38: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Type, 2021 and 2031
Figure 39: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Type, 2022–2031
Figure 40: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2021 and 2031
Figure 41: Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 42: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, 2017–2031
Figure 43: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 44: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 45: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2021 and 2031
Figure 46: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 47: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Type, 2021 and 2031
Figure 48: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Type, 2022–2031
Figure 49: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2021 and 2031
Figure 50: Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 51: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, 2017–2031
Figure 52: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 53: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2021-2031
Figure 54: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2021 and 2031
Figure 55: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 56: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Type, 2021 and 2031
Figure 57: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Type, 2022–2031
Figure 58: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2021 and 2031
Figure 59: Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 60: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) Forecast, 2017–2031
Figure 61: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 62: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2021-2031
Figure 63: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2021 and 2031
Figure 64: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2022–2031
Figure 65: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Type, 2021 and 2031
Figure 66: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Type, 2022–2031
Figure 67: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2021 and 2031
Figure 68: Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2022–2031
Figure 69: Company Share Analysis, 2021