Reports
Reports
Analyst’s Viewpoint regarding Cancer Diagnostics Market Scenario
Drivers of the cancer diagnostics market include growing cases of cancer globally, increasing demand for early detection, technological advancements in liquid biopsy, molecular diagnostics, and AI-based imaging. Government policies, expanding applications of precision medicine and expanding awareness also contribute to market expansion.
Yet, high costs of advanced diagnostics, restrictive reimbursement policies, and regulatory hurdles are the major impediments to it, coupled with uneven access to diagnostic centers in low- and middle-income nations. In spite of these constraints, the frontiers of the fusion of AI and machine learning toward precise diagnostics, creation of non-invasive testing techniques, and biomarker-based diagnostic investigations will drive early diagnosis and tailor-made treatment techniques.
Cancer diagnosis implies the identification, detection, and confirmation of cancer within the body through the execution of different medical tests and imaging tests. It includes laboratory tests (like biopsy, blood tests, and molecular diagnostics), imaging techniques (like MRI, CT scan, PET scan, and ultrasound), and sophisticated technologies such as liquid biopsy and genetic testing to identify the type, stage, and extent of cancer. Timely and accurate diagnosis of cancer is a critical factor for treatment planning, enhancing patient outcomes, and facilitating precision medicine strategies.
| Attribute | Detail |
|---|---|
| Cancer Diagnostics Market Drivers |
|
The growth of the cancer diagnostics market is expected to be driven by technological advancements in cancer diagnostics products. Advanced technologies like next-generation sequencing (NGS), liquid biopsies, advanced imaging technologies, etc. are improving the rate of cancer detection, accuracy, and speed. Apart from high detection rates, they facilitate early diagnosis and accuracy in the process of disease identification, which is useful for effective treatment
For instance, in October 2024, Owkin, the first end-to-end AI-biotech that uses cutting-edge causal AI to unlock precision drug discovery, development, and diagnostics, announced MSIntuit CRC v21, a next-generation AI solution. Aimed at transforming the detection and treatment of colorectal cancer (CRC), MSIntuit CRC v2 will initially launch as an RUO version in the US on Roche’s navify Digital Pathology enterprise software.
Increasing awareness and early diagnosis are expected to drive the growth of cancer diagnostics Industry. With growing awareness among doctors and the general population regarding the importance of early cancer detection, people are increasingly turning to routine check-ups and diagnostic procedures. Enhanced awareness is driven by a multitude of factors such as public awareness campaigns, training programs, and sharing information via electronic and print media
Early diagnosis is required as it allows for greater scope for less invasive but more impactful treatment, which ultimately means a higher patient outcome and rate of survival. Keeping this in mind, more advanced diagnostic equipment and devices in the way of advanced imaging, i.e., high-end MRI, CT, PET/CT, next-generation sequencing (NGS), liquid biopsies, and so on, machines, and technologies are now required
These technologies not only improve the efficiency and accuracy of cancer diagnosis but also allow cancer to be diagnosed at earlier stages, when it is the most curable. The synergy between growing awareness and the availability of more advanced diagnostic equipment is fueling the market for cancer diagnostics, and it is a growth- and innovation-driven sector of the healthcare industry.
Antiplatelet medications are dominating the market owing to their essential role in treatment of the disease, which is identified with constricted arteries that hinder limb blood supply. Of the various drug types, aspirin is most widely used due to its efficacy in cardiovascular event prevention in peripheral arterial disease (PAD).
However, more effective drugs like clopidogrel and ticagrelor also are becoming popular, with improved platelet inhibition and improved patient outcomes with more extensive disease. Rising incidence of PAD, fueled by risk factors like diabetes mellitus, smoking, and aging population, has accelerated the need for potent antiplatelet therapy. Additionally, clinical trials are also in process to assess the advantages of double antiplatelet therapy in enhancing patient outcomes, which is propelling the market growth further.
Since medical professionals increasingly concentrate on integrated treatment approaches to manage symptoms and avoid complications among PAD patients, antiplatelet medications will also remain an important treatment part for managing this disease.
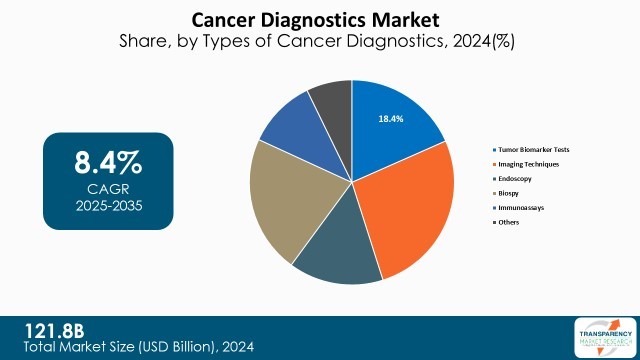
Imaging techniques lead the cancer diagnostics market, as they are non-invasive and easily available, with capability to identify tumors at any stage. Techniques including MRI, CT scan, PET scan, and ultrasound contribute to early detection of cancer and cancer staging, as well as tracking cancer therapy.
Biopsy holds the second-largest market share due to its definitive role in confirming cancer presence. Biopsy tests such as needle biopsy, liquid biopsy, and surgical biopsy yield histopathological and molecular information required for treatment tailoring.
Rising adoption of liquid biopsy, whose less invasive method for the detection of circulating tumor DNA (ctDNA) and the other biomarkers, is driving the market for this segment predominantly
| Attribute | Detail |
|---|---|
| Leading Region | North America |
North America is the leading region in the cancer diagnostics Industry due to its well-established healthcare infrastructure, high rate of adoption of advanced diagnostic technologies, and robust government programs for the early detection of cancer. The region boasts high R&D expenditure, high level of key players' concentration, and extensive availability of advanced diagnostic technologies like liquid biopsy, next-generation sequencing (NGS), and AI-driven imaging solutions
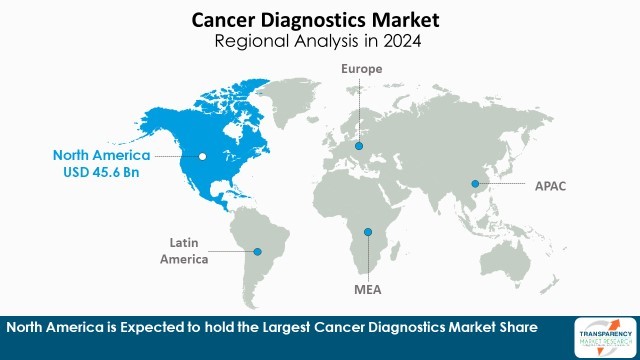
Furthermore, the region's high cancer prevalence, increasing knowledge of early detection, and favorable reimbursement policies - all contribute to strengthening the region's market leadership. With continued innovation in precision medicine and biomarker-based diagnostics, North America will most likely remain at the top of the global cancer diagnostics market
Cancer Diagnostics, Inc., Abbott, Bio-Rad Laboratories, Inc., GE HealthCare, Thermo Fisher Scientific, Inc., F. Hoffmann-La Roche AG, Quest Diagnostics Incorporated, QIAGEN, Siemens Healthineers, MedGenome, and Biodesix are some of the leading key players operating in the global cancer diagnostics market.
Each of these players have been have been profiled in the cancer diagnostics market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
Key Developments
| Attribute | Detail |
|---|---|
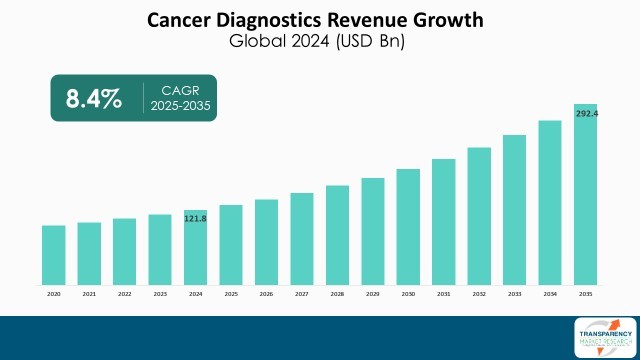
| Size in 2024 | US$ 121.8 Bn |
| Forecast Value in 2035 | More than US$ 292.4 Bn |
| CAGR | 8.4% |
| Forecast Period | 2025 to 2035 |
| Historical Data Available for | 2020 to 2024 |
| Quantitative Units | US$ Mn/Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
The cancer diagnostics market was valued at US$ 121.8 Bn in 2024
The cancer diagnostics market business is projected to cross US$ 292.4 Bn by the end of 2035
Technological advancements in cancer diagnostics products, increasing awareness and early detection, and government initiatives and funding
The CAGR is anticipated to be 8.4% from 2025 to 2035
North America is expected to account for the largest share from 2025 to 2035
Cancer Diagnostics, Inc., Abbott, Bio-Rad Laboratories, Inc., GE HealthCare, Thermo Fisher Scientific, Inc., F. Hoffmann-La Roche AG, Quest Diagnostics Incorporated, QIAGEN, Siemens Healthineers, MedGenome, and Biodesix
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Cancer Diagnostics Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.1.2. Industry Evolution/Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Cancer Diagnostics Market Analysis and Forecasts, 2020 - 2035
4.4.1. Market Revenue Projections (US$ Bn)
5. Key Insights
5.1. Global Cancer Incidence 2022
5.2. Key Industry Events
5.3. Regulatory Landscape across Key Regions / Countries
5.4. Key Purchase Metrics for End-Users
5.5. Brand Analysis
5.6. A New Artificial Intelligence Tool for Cancer
5.7. Brand Analysis
5.8. Emerging Technologies in Early Cancer Detection
6. Global Cancer Diagnostics Market Analysis and Forecasts, By Types of Cancer Diagnostics
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast By Types of Cancer Diagnostics, 2020 - 2035
6.3.1. Tumor Biomarker Tests
6.3.2. Imaging Techniques
6.3.3. Endoscopy
6.3.4. Biopsy
6.3.5. Immunoassays
6.3.6. Others
6.4. Market Attractiveness By Types of Cancer Diagnostics
7. Global Cancer Diagnostics Market Analysis and Forecasts, By Product Type
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast By Product Type, 2020 - 2035
7.3.1. Instruments
7.3.2. Assay Kits & Reagent
7.4. Market Attractiveness By Product Type
8. Global Cancer Diagnostics Market Analysis and Forecasts, By Application
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast By Application, 2020 - 2035
8.3.1. Lung Cancer
8.3.2. Breast Cancer
8.3.3. Colorectal Cancer
8.3.4. Prostate Cancer
8.3.5. Liver Cancer
8.3.6. Ovarian Cancer
8.3.7. Kidney Cancer
8.3.8. Pancreatic Cancer
8.3.9. Blood Cancer
8.3.10. Others
8.4. Market Attractiveness By Application
9. Global Cancer Diagnostics Market Analysis and Forecasts, By End-user
9.1. Introduction & Definition
9.2. Key Findings / Developments
9.3. Market Value Forecast By End-user, 2020 - 2035
9.3.1. Hospitals
9.3.2. Diagnostics Centers
9.3.3. At Home Settings
9.3.4. Others
9.4. Market Attractiveness By End-user
10. Global Cancer Diagnostics Market Analysis and Forecasts, By Region
10.1. Key Findings
10.2. Market Value Forecast By Region
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Market Attractiveness By Country/Region
11. North America Cancer Diagnostics Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast By Types of Cancer Diagnostics, 2020 - 2035
11.2.1. Tumor Biomarker Tests
11.2.2. Imaging Techniques
11.2.3. Endoscopy
11.2.4. Biopsy
11.2.5. Immunoassays
11.2.6. Others
11.3. Market Value Forecast By Product Type, 2020 - 2035
11.3.1. Instruments
11.3.2. Assay Kits & Reagent
11.4. Market Value Forecast By Application, 2020 - 2035
11.4.1. Lung Cancer
11.4.2. Breast Cancer
11.4.3. Colorectal Cancer
11.4.4. Prostate Cancer
11.4.5. Liver Cancer
11.4.6. Ovarian Cancer
11.4.7. Kidney Cancer
11.4.8. Pancreatic Cancer
11.4.9. Blood Cancer
11.4.10. Others
11.5. Market Value Forecast By End-user, 2020 - 2035
11.5.1. Hospitals
11.5.2. Diagnostics Centers
11.5.3. At Home Settings
11.5.4. Others
11.6. Market Value Forecast By Country, 2020 - 2035
11.6.1. U.S.
11.6.2. Canada
11.7. Market Attractiveness Analysis
11.7.1. By Types of Cancer Diagnostics
11.7.2. By Product Type
11.7.3. By Application
11.7.4. By End-user
11.7.5. By Country
12. Europe Cancer Diagnostics Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast By Types of Cancer Diagnostics, 2020 - 2035
12.2.1. Tumor Biomarker Tests
12.2.2. Imaging Techniques
12.2.3. Endoscopy
12.2.4. Biopsy
12.2.5. Immunoassays
12.2.6. Others
12.3. Market Value Forecast By Product Type, 2020 - 2035
12.3.1. Instruments
12.3.2. Assay Kits & Reagent
12.4. Market Value Forecast By Application, 2020 - 2035
12.4.1. Lung Cancer
12.4.2. Breast Cancer
12.4.3. Colorectal Cancer
12.4.4. Prostate Cancer
12.4.5. Liver Cancer
12.4.6. Ovarian Cancer
12.4.7. Kidney Cancer
12.4.8. Pancreatic Cancer
12.4.9. Blood Cancer
12.4.10. Others
12.5. Market Value Forecast By End-user, 2020 - 2035
12.5.1. Hospitals
12.5.2. Diagnostics Centers
12.5.3. At Home Settings
12.5.4. Others
12.6. Market Value Forecast By Country/Sub-region, 2020 - 2035
12.6.1. Germany
12.6.2. U.K.
12.6.3. France
12.6.4. Spain
12.6.5. Italy
12.6.6. Rest of Europe
12.7. Market Attractiveness Analysis
12.7.1. By Types of Cancer Diagnostics
12.7.2. By Product Type
12.7.3. By Application
12.7.4. By End-user
12.7.5. By Country/Sub-region
13. Asia Pacific Cancer Diagnostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast By Types of Cancer Diagnostics, 2020 - 2035
13.2.1. Tumor Biomarker Tests
13.2.2. Imaging Techniques
13.2.3. Endoscopy
13.2.4. Biopsy
13.2.5. Immunoassays
13.2.6. Others
13.3. Market Value Forecast By Product Type, 2020 - 2035
13.3.1. Instruments
13.3.2. Assay Kits & Reagent
13.4. Market Value Forecast By Application, 2020 - 2035
13.4.1. Lung Cancer
13.4.2. Breast Cancer
13.4.3. Colorectal Cancer
13.4.4. Prostate Cancer
13.4.5. Liver Cancer
13.4.6. Ovarian Cancer
13.4.7. Kidney Cancer
13.4.8. Pancreatic Cancer
13.4.9. Blood Cancer
13.4.10. Others
13.5. Market Value Forecast By End-user, 2020 - 2035
13.5.1. Hospitals
13.5.2. Diagnostics Centers
13.5.3. At Home Settings
13.5.4. Others
13.6. Market Value Forecast By Country/Sub-region, 2020 - 2035
13.6.1. China
13.6.2. Japan
13.6.3. India
13.6.4. Australia & New Zealand
13.6.5. Rest of Asia Pacific
13.7. Market Attractiveness Analysis
13.7.1. By Types of Cancer Diagnostics
13.7.2. By Product Type
13.7.3. By Application
13.7.4. By End-user
13.7.5. By Country/Sub-region
14. Latin America Cancer Diagnostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast By Types of Cancer Diagnostics, 2020 - 2035
14.2.1. Tumor Biomarker Tests
14.2.2. Imaging Techniques
14.2.3. Endoscopy
14.2.4. Biopsy
14.2.5. Immunoassays
14.2.6. Others
14.3. Market Value Forecast By Product Type, 2020 - 2035
14.3.1. Instruments
14.3.2. Assay Kits & Reagent
14.4. Market Value Forecast By Application, 2020 - 2035
14.4.1. Lung Cancer
14.4.2. Breast Cancer
14.4.3. Colorectal Cancer
14.4.4. Prostate Cancer
14.4.5. Liver Cancer
14.4.6. Ovarian Cancer
14.4.7. Kidney Cancer
14.4.8. Pancreatic Cancer
14.4.9. Blood Cancer
14.4.10. Others
14.5. Market Value Forecast By End-user, 2020 - 2035
14.5.1. Hospitals
14.5.2. Diagnostics Centers
14.5.3. At Home Settings
14.5.4. Others
14.6. Market Value Forecast By Country/Sub-region, 2020 - 2035
14.6.1. Brazil
14.6.2. Mexico
14.6.3. Rest of Latin America
14.7. Market Attractiveness Analysis
14.7.1. By Types of Cancer Diagnostics
14.7.2. By Product Type
14.7.3. By Application
14.7.4. By End-user
14.7.5. By Country/Sub-region
15. Middle East & Africa Cancer Diagnostics Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast By Types of Cancer Diagnostics, 2020 - 2035
15.2.1. Tumor Biomarker Tests
15.2.2. Imaging Techniques
15.2.3. Endoscopy
15.2.4. Biopsy
15.2.5. Immunoassays
15.2.6. Others
15.3. Market Value Forecast By Product Type, 2020 - 2035
15.3.1. Instruments
15.3.2. Assay Kits & Reagent
15.4. Market Value Forecast By Application, 2020 - 2035
15.4.1. Lung Cancer
15.4.2. Breast Cancer
15.4.3. Colorectal Cancer
15.4.4. Prostate Cancer
15.4.5. Liver Cancer
15.4.6. Ovarian Cancer
15.4.7. Kidney Cancer
15.4.8. Pancreatic Cancer
15.4.9. Blood Cancer
15.4.10. Others
15.5. Market Value Forecast By End-user, 2020 - 2035
15.5.1. Hospitals
15.5.2. Diagnostics Centers
15.5.3. At Home Settings
15.5.4. Others
15.6. Market Value Forecast By Country/Sub-region, 2020 - 2035
15.6.1. GCC Countries
15.6.2. South Africa
15.6.3. Rest of Middle East & Africa
15.7. Market Attractiveness Analysis
15.7.1. By Types of Cancer Diagnostics
15.7.2. By Product Type
15.7.3. By Application
15.7.4. By End-user
15.7.5. By Country/Sub-region
16. Competition Landscape
16.1. Market Player - Competition Matrix (By Tier and Size of companies)
16.2. Market Share Analysis By Company (2024)
16.3. Company Profiles
16.3.1. Cancer Diagnostics, Inc.
16.3.1.1. Company Overview
16.3.1.2. Financial Overview
16.3.1.3. Product Portfolio
16.3.1.4. Business Strategies
16.3.1.5. Recent Developments
16.3.2. Abbott
16.3.2.1. Company Overview
16.3.2.2. Financial Overview
16.3.2.3. Product Portfolio
16.3.2.4. Business Strategies
16.3.2.5. Recent Developments
16.3.3. Bio-Rad Laboratories, Inc.
16.3.3.1. Company Overview
16.3.3.2. Financial Overview
16.3.3.3. Product Portfolio
16.3.3.4. Business Strategies
16.3.3.5. Recent Developments
16.3.4. GE HealthCare
16.3.4.1. Company Overview
16.3.4.2. Financial Overview
16.3.4.3. Product Portfolio
16.3.4.4. Business Strategies
16.3.4.5. Recent Developments
16.3.5. Thermo Fisher Scientific, Inc.
16.3.5.1. Company Overview
16.3.5.2. Financial Overview
16.3.5.3. Product Portfolio
16.3.5.4. Business Strategies
16.3.5.5. Recent Developments
16.3.6. F. Hoffmann-La Roche AG
16.3.6.1. Company Overview
16.3.6.2. Financial Overview
16.3.6.3. Product Portfolio
16.3.6.4. Business Strategies
16.3.6.5. Recent Developments
16.3.7. Quest Diagnostics Incorporated
16.3.7.1. Company Overview
16.3.7.2. Financial Overview
16.3.7.3. Product Portfolio
16.3.7.4. Business Strategies
16.3.7.5. Recent Developments
16.3.8. QIAGEN
16.3.8.1. Company Overview
16.3.8.2. Financial Overview
16.3.8.3. Product Portfolio
16.3.8.4. Business Strategies
16.3.8.5. Recent Developments
16.3.9. Siemens Healthineers
16.3.9.1. Company Overview
16.3.9.2. Financial Overview
16.3.9.3. Product Portfolio
16.3.9.4. Business Strategies
16.3.9.5. Recent Developments
16.3.10. MedGenome
16.3.10.1. Company Overview
16.3.10.2. Financial Overview
16.3.10.3. Product Portfolio
16.3.10.4. Business Strategies
16.3.10.5. Recent Developments
16.3.11. Biodesix
16.3.11.1. Company Overview
16.3.11.2. Financial Overview
16.3.11.3. Product Portfolio
16.3.11.4. Business Strategies
16.3.11.5. Recent Developments
List of Tables
Table 01: Global Cancer Diagnostics Market Value (US$ Bn) Forecast, By Type of Cancer Diagnostics, 2020-2035
Table 02: Global Cancer Diagnostics Market Value (US$ Bn) Forecast, By Product Type, 2020-2035
Table 03: Global Cancer Diagnostics Market Value (US$ Bn) Forecast, By Application, 2020-2035
Table 04: Global Cancer Diagnostics Market Value (US$ Bn) Forecast, By End-user, 2020-2035
Table 05: Global Cancer Diagnostics Market Value (US$ Bn) Forecast, By Region, 2020-2035
Table 06: North America - Cancer Diagnostics Market Value (US$ Bn) Forecast, by Country, 2020-2035
Table 07: North America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Type of Cancer Diagnostics, 2020-2035
Table 08: North America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Product Type, 2020-2035
Table 09: North America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Application, 2020-2035
Table 10: North America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By End-user, 2020-2035
Table 11: Europe - Cancer Diagnostics Market Value (US$ Bn) Forecast, by Country / Sub-region, 2020-2035
Table 12: Europe - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Type of Cancer Diagnostics, 2020-2035
Table 13: Europe - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Product Type, 2020-2035
Table 14: Europe - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Application, 2020-2035
Table 15: Europe - Cancer Diagnostics Market Value (US$ Bn) Forecast, By End-user, 2020-2035
Table 16: Asia Pacific - Cancer Diagnostics Market Value (US$ Bn) Forecast, by Country / Sub-region, 2020-2035
Table 17: Asia Pacific - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Type of Cancer Diagnostics, 2020-2035
Table 18: Asia Pacific - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Product Type, 2020-2035
Table 19: Asia Pacific - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Application, 2020-2035
Table 20: Asia Pacific - Cancer Diagnostics Market Value (US$ Bn) Forecast, By End-user, 2020-2035
Table 21: Latin America - Cancer Diagnostics Market Value (US$ Bn) Forecast, by Country / Sub-region, 2020-2035
Table 22: Latin America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Type of Cancer Diagnostics, 2020-2035
Table 23: Latin America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Product Type, 2020-2035
Table 24: Latin America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Application, 2020-2035
Table 25: Latin America - Cancer Diagnostics Market Value (US$ Bn) Forecast, By End-user, 2020-2035
Table 26: Middle East & Africa - Cancer Diagnostics Market Value (US$ Bn) Forecast, by Country / Sub-region, 2020-2035
Table 27: Middle East & Africa - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Type of Cancer Diagnostics, 2020-2035
Table 28: Middle East & Africa - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Product Type, 2020-2035
Table 29: Middle East & Africa - Cancer Diagnostics Market Value (US$ Bn) Forecast, By Application, 2020-2035
Table 30: Middle East & Africa - Cancer Diagnostics Market Value (US$ Bn) Forecast, By End-user, 2020-2035
List of Figures
Figure 01: Global Cancer Diagnostics Market Value Share Analysis, By Type of Cancer Diagnostics, 2024 and 2035
Figure 02: Global Cancer Diagnostics Market Attractiveness Analysis, By Type of Cancer Diagnostics, 2025-2035
Figure 03: Global Cancer Diagnostics Market Revenue (US$ Bn), by Tumor Biomarker Tests, 2020-2035
Figure 04: Global Cancer Diagnostics Market Revenue (US$ Bn), by Imaging Techniques, 2020-2035
Figure 05: Global Cancer Diagnostics Market Revenue (US$ Bn), by Endoscopy, 2020-2035
Figure 06: Global Cancer Diagnostics Market Revenue (US$ Bn), by Biopsy, 2020-2035
Figure 07: Global Cancer Diagnostics Market Revenue (US$ Bn), by Immunoassays, 2020-2035
Figure 08: Global Cancer Diagnostics Market Revenue (US$ Bn), by Others, 2020-2035
Figure 09: Global Cancer Diagnostics Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 10: Global Cancer Diagnostics Market Attractiveness Analysis, By Product Type, 2025-2035
Figure 11: Global Cancer Diagnostics Market Revenue (US$ Bn), by Instruments, 2020-2035
Figure 12: Global Cancer Diagnostics Market Revenue (US$ Bn), by Assay Kits & Reagent, 2020-2035
Figure 13: Global Cancer Diagnostics Market Value Share Analysis, By Application, 2024 and 2035
Figure 14: Global Cancer Diagnostics Market Attractiveness Analysis, By Application, 2025-2035
Figure 15: Global Cancer Diagnostics Market Revenue (US$ Bn), by Lung Cancer, 2020-2035
Figure 16: Global Cancer Diagnostics Market Revenue (US$ Bn), by Breast Cancer, 2020-2035
Figure 17: Global Cancer Diagnostics Market Revenue (US$ Bn), by Colorectal Cancer, 2020-2035
Figure 18: Global Cancer Diagnostics Market Revenue (US$ Bn), by Prostate Cancer, 2020-2035
Figure 19: Global Cancer Diagnostics Market Revenue (US$ Bn), by Liver Cancer, 2020-2035
Figure 20: Global Cancer Diagnostics Market Revenue (US$ Bn), by Ovarian Cancer, 2020-2035
Figure 21: Global Cancer Diagnostics Market Revenue (US$ Bn), by Kidney Cancer, 2020-2035
Figure 22: Global Cancer Diagnostics Market Revenue (US$ Bn), by Pancreatic Cancer, 2020-2035
Figure 23: Global Cancer Diagnostics Market Revenue (US$ Bn), by Blood Cancer, 2020-2035
Figure 24: Global Cancer Diagnostics Market Revenue (US$ Bn), by Others, 2020-2035
Figure 25: Global Cancer Diagnostics Market Value Share Analysis, By End-user, 2024 and 2035
Figure 26: Global Cancer Diagnostics Market Attractiveness Analysis, By End-user, 2025-2035
Figure 27: Global Cancer Diagnostics Market Revenue (US$ Bn), by Hospitals, 2020-2035
Figure 28: Global Cancer Diagnostics Market Revenue (US$ Bn), by Diagnostic Centers, 2020-2035
Figure 29: Global Cancer Diagnostics Market Revenue (US$ Bn), by At-Home Settings, 2020-2035
Figure 30: Global Cancer Diagnostics Market Revenue (US$ Bn), by Others, 2020-2035
Figure 31: Global Cancer Diagnostics Market Value Share Analysis, By Region, 2024 and 2035
Figure 32: Global Cancer Diagnostics Market Attractiveness Analysis, By Region, 2025-2035
Figure 33: North America - Cancer Diagnostics Market Value (US$ Bn) Forecast, 2020-2035
Figure 34: North America - Cancer Diagnostics Market Value Share Analysis, by Country, 2024 and 2035
Figure 35: North America - Cancer Diagnostics Market Attractiveness Analysis, by Country, 2025-2035
Figure 36: North America - Cancer Diagnostics Market Value Share Analysis, By Type of Cancer Diagnostics, 2024 and 2035
Figure 37: North America - Cancer Diagnostics Market Attractiveness Analysis, By Type of Cancer Diagnostics, 2025-2035
Figure 38: North America - Cancer Diagnostics Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 39: North America - Cancer Diagnostics Market Attractiveness Analysis, By Product Type, 2025-2035
Figure 40: North America - Cancer Diagnostics Market Value Share Analysis, By Application, 2024 and 2035
Figure 41: North America - Cancer Diagnostics Market Attractiveness Analysis, By Application, 2025-2035
Figure 42: North America - Cancer Diagnostics Market Value Share Analysis, By End-user, 2024 and 2035
Figure 43: North America - Cancer Diagnostics Market Attractiveness Analysis, By End-user, 2025-2035
Figure 44: Europe - Cancer Diagnostics Market Value (US$ Bn) Forecast, 2020-2035
Figure 45: Europe - Cancer Diagnostics Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 46: Europe - Cancer Diagnostics Market Attractiveness Analysis, by Country / Sub-region, 2025-2035
Figure 47: Europe - Cancer Diagnostics Market Value Share Analysis, By Type of Cancer Diagnostics, 2024 and 2035
Figure 48: Europe - Cancer Diagnostics Market Attractiveness Analysis, By Type of Cancer Diagnostics, 2025-2035
Figure 49: Europe - Cancer Diagnostics Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 50: Europe - Cancer Diagnostics Market Attractiveness Analysis, By Product Type, 2025-2035
Figure 51: Europe - Cancer Diagnostics Market Value Share Analysis, By Application, 2024 and 2035
Figure 52: Europe - Cancer Diagnostics Market Attractiveness Analysis, By Application, 2025-2035
Figure 53: Europe - Cancer Diagnostics Market Value Share Analysis, By End-user, 2024 and 2035
Figure 54: Europe - Cancer Diagnostics Market Attractiveness Analysis, By End-user, 2025-2035
Figure 55: Asia Pacific - Cancer Diagnostics Market Value (US$ Bn) Forecast, 2020-2035
Figure 56: Asia Pacific - Cancer Diagnostics Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 57: Asia Pacific - Cancer Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2025-2035
Figure 58: Asia Pacific - Cancer Diagnostics Market Value Share Analysis, By Type of Cancer Diagnostics, 2024 and 2035
Figure 59: Asia Pacific - Cancer Diagnostics Market Attractiveness Analysis, By Type of Cancer Diagnostics, 2025-2035
Figure 60: Asia Pacific - Cancer Diagnostics Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 61: Asia Pacific - Cancer Diagnostics Market Attractiveness Analysis, By Product Type, 2025-2035
Figure 62: Asia Pacific - Cancer Diagnostics Market Value Share Analysis, By Application, 2024 and 2035
Figure 63: Asia Pacific - Cancer Diagnostics Market Attractiveness Analysis, By Application, 2025-2035
Figure 64: Asia Pacific - Cancer Diagnostics Market Value Share Analysis, By End-user, 2024 and 2035
Figure 65: Asia Pacific - Cancer Diagnostics Market Attractiveness Analysis, By End-user, 2025-2035
Figure 66: Latin America - Cancer Diagnostics Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 67: Latin America - Cancer Diagnostics Market Attractiveness Analysis, by Country / Sub-region, 2025-2035
Figure 68: Latin America - Cancer Diagnostics Market Value Share Analysis, By Type of Cancer Diagnostics, 2024 and 2035
Figure 69: Latin America - Cancer Diagnostics Market Attractiveness Analysis, By Type of Cancer Diagnostics, 2025-2035
Figure 70: Latin America - Cancer Diagnostics Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 71: Latin America - Cancer Diagnostics Market Attractiveness Analysis, By Product Type, 2025-2035
Figure 72: Latin America - Cancer Diagnostics Market Value Share Analysis, By Application, 2024 and 2035
Figure 73: Latin America - Cancer Diagnostics Market Attractiveness Analysis, By Application, 2025-2035
Figure 74: Latin America - Cancer Diagnostics Market Value Share Analysis, By End-user, 2024 and 2035
Figure 75: Latin America - Cancer Diagnostics Market Attractiveness Analysis, By End-user, 2025-2035
Figure 76: Middle East & Africa - Cancer Diagnostics Market Value Share Analysis, by Country / Sub-region, 2024 and 2035
Figure 77: Middle East & Africa - Cancer Diagnostics Market Attractiveness Analysis, by Country / Sub-region, 2025-2035
Figure 78: Middle East & Africa - Cancer Diagnostics Market Value Share Analysis, By Type of Cancer Diagnostics, 2024 and 2035
Figure 79: Middle East & Africa - Cancer Diagnostics Market Attractiveness Analysis, By Type of Cancer Diagnostics, 2025-2035
Figure 80: Middle East & Africa - Cancer Diagnostics Market Value Share Analysis, By Product Type, 2024 and 2035
Figure 81: Middle East & Africa - Cancer Diagnostics Market Attractiveness Analysis, By Product Type, 2025-2035
Figure 82: Middle East & Africa - Cancer Diagnostics Market Value Share Analysis, By Application, 2024 and 2035
Figure 83: Middle East & Africa - Cancer Diagnostics Market Attractiveness Analysis, By Application, 2025-2035
Figure 84: Middle East & Africa - Cancer Diagnostics Market Value Share Analysis, By End-user, 2024 and 2035
Figure 85: Middle East & Africa - Cancer Diagnostics Market Attractiveness Analysis, By End-user, 2025-2035