Reports
Reports
As per the business intelligence report by the Transparency Market Research, the global biological safety testing market is expected to arise from its last evaluation of US$ 1.31 billion at the beginning of the forecast period i.e. 2015 to hit the mark of US$ 3.08 billion at the end of the forecast period i.e. in 2024. The research report also predicts that the global biological safety testing market will expand at a stellar growth rate of 10 % over the forecast period of 2016 to 2024. Continuous developments in terms of assistive technologies as well as the rising geriatric population are two of the major driving factors influencing the growth within the global biological safety testing market.
The rising usage of biological safety testing in various end use industry verticals such as biotechnology as well as pharmaceutical industries, is anticipated to cater several new opportunities for the global biological safety testing market. Furthermore, the rise in the number of elderly population that is more prone to various severe health problems and chronic health conditions as well as the increasing number and instances of cancer patients across the world are also expected to positively influence the demand dynamics within the global biological safety testing market in coming years.
The types of products offered by the players and vendors functional within the global biological safety testing market include reagents and kits as well as instrument, including autoclaves, biological safety cabinet (such as class 1, class 2, and class 3), and laboratory centrifuges, among others). Among these, the instruments segment dominated the global biological safety testing market, holding around 29.2 % of the total industry share in 2015. However, the increasing focus on the development of the kits and reagents, owing to their rising popularity as well as launch of new, innovative, and enhanced technologies by major players is also fueling the demand within the reagents and kits segment in the global biological safety testing market.
Types of tests offered by the vendors in the global biological safety testing market are sterility tests, bioburden tests, adventitious agent detection tests, stability tests, endotoxin tests, cell line authentication and characterization tests, residual host contaminant detection tests, and toxicity tests, among others. Key applications for the products and solutions in the global biological safety testing market include blood and blood products, stem cell products, cellular therapy, vaccines and therapeutics, tissue and tissue products, and gene therapy. According to application, the blood and blood products segment dominated the global biological safety testing market in recent past, accounting for almost 52.7 % of the total industry share in the year 2015.
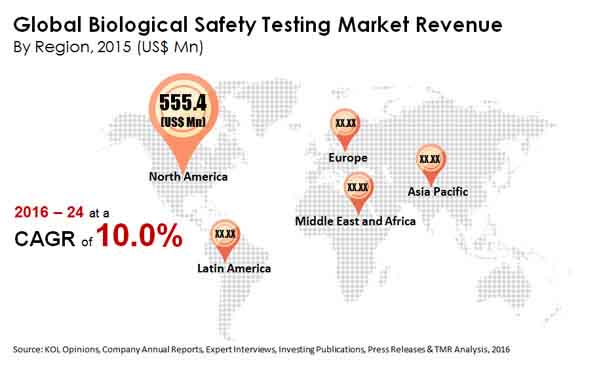
Leading regions and nations functional within the landscape of the global biological safety testing market include Europe (including France, Italy, Germany, Spain, the United Kingdom, and rest of the Europe), Latin America (including Brazil, Mexico, and rest of the Latin America), North America (including Canada and the United States), Middle East and Africa (including South Africa, Saudi Arabia, and rest of the Middle East and Africa), and Asia Pacific (including China, Japan, India, and rest of the Asia Pacific). Geographically, North America emerged as the leading regions within the global biological safety testing market. On the other hand, emerging economies in Latin America are also expected to create various new opportunities for expansion within the global biological safety testing market in coming years.
Major factors influencing the growth within the global biological safety testing market in North America include massive number of leading incumbent players operating within the region as well as presence of technologically advanced and well-trenched healthcare and medical infrastructure in the region. The research report projects that the North America region accounts for the largest i.e. 42.2 % of the total share in the global biological safety testing market. Second largest share in the global biological safety testing market can be attributed to the Europe region. The Asia Pacific is yet another region displaying considerable growth opportunities within the global biological safety testing market. Moreover, the report predicts that the global biological safety testing market will grow at the highest rate over the forecast period in Asia Pacific.
The competitive landscape of the global biological safety testing market is considered to be fragmented, owing to a large number of notable players and vendors operational within the industry. Some of the most prominent players and manufacturers operational within the global biological safety testing market include Charles River Laboratories International, Inc., Lonza Group, SGS SA, Toxikon, Inc., Paragon Bioservices, Inc., Thermo Fisher Scientific Inc., Merck KGaA, Avance Biosciences, WuXi PharmaTech (Cayman) Inc., NuAire, and Nordic Scientific & Natural Solutions AB.
Leading players and vendors functional within the global biological safety testing market are engaged in constant development of new and innovative assistive technologies. This is resulting in the rising cash in-flow aimed at fueling the research and development projects in the global biological safety testing market. Moreover, various leading players within the global biological safety testing market are focusing on regional expansion, as well as utilizing and adopting several corporate strategies such as mergers and acquisitions, collaborations, and strategic partnerships.
Biological safety testing market is projected to reach US$3.08 bn by 2024
Biological safety testing market to Expand at a CAGR of 10.0% from 2016 to 2024
Biological safety testing market is driven by increasing number and instances of cancer patients across the world
North America accounted for a major share of the global biological safety testing market
Key players in the biological safety testing market include Charles River Laboratories International, Inc., Lonza Group, SGS SA, Toxikon, Inc., Paragon Bioservices, Inc., Thermo Fisher Scientific Inc
Section 1 Preface
1.1 Report Scope and Market Segmentation
1.2 Research Highlights
Section-2 Assumptions and Research Methodology
2.1 Assumptions and Acronyms Used
2.2 Research Methodology
Section-3 Executive Summary
3.1 Global Biological Safety Testing Market: Market Snapshot
3.2 Global Biological Safety Testing Market: Opportunity Map
Section-4 Market Overview
4.1 Global Biological Safety Testing Market: Product Overview
4.2 Global Biological Safety Testing Market: Key Industry Developments
Section-5 Market Dynamics
5.1 Drivers and Restraints Snapshot Analysis
5.1.1 Drivers
5.1.1.1 Growing biopharmaceutical industry
5.1.1.2 Increasing prevalence of chronic diseases
5.1.1.3 Improving health care infrastructure
5.1.1.4 Technological advancements
5.1.1.5 Increasing government initiatives
5.1.1.6 Rising drug development
5.1.2 Restraints
5.1.2.1 High health care cost
5.1.2.2 Regulatory scenario
5.1.2.3 Lack of trained professionals
5.1.3 Opportunity
5.1.3.1 Technological development and innovation
5.1.3.2 Expansion in emerging economies such as countries in Asia Pacific, South Africa, and Brazil
5.2 Opportunity Analysis
5.3 Key Trends
5.4 Global Biological Safety Testing Market Revenue Forecast
5.5 Porter’s Five Forces Analysis
5.6 Global Biological Safety Testing Market Outlook
Section-6 Global Biological Safety Testing Market Analysis, by Product Type
6.1 Key Findings
6.2 Introduction
6.3 Global Biological Safety Testing Market Value Share Analysis, by Product Type
6.4 Global Biological Safety Testing Market Forecast, by Product Type
6.4.1 Instruments
6.4.2 Reagents & Kits
6.5 Global Biological Safety Testing Market Attractiveness Analysis
Section-7 Biological Safety Testing Market Analysis, by Test Type
7.1 Key Findings
7.2 Introduction
7.3 Global Biological Safety Testing Market Value Share Analysis, by Test Type
7.4 Global Biological Safety Testing Market Forecast, by Test Type
7.4.1 Endotoxin Tests
7.4.2 Sterility Tests
7.4.3 Cell Line Authentication and Characterization Tests
7.4.4 Bioburden Tests
7.4.5 Residual Host Contaminant Detection Tests
7.4.6 Adventitious Agent Detection Tests
7.4.7 Other Tests (Toxicity tests, Stability tests, etc.)
Section-8 Biological Safety Testing Market Analysis, by Test Type
8.1 Key Findings
8.2 Introduction
8.3 Global Biological Safety Testing Market Value Share Analysis, by Application Type
8.4 Global Biological Safety Testing Market Forecast, by Application Type
8.4.1 Vaccines and Therapeutics
8.4.2 Blood and Blood Products
8.4.3 Cellular Therapy
8.4.4 Gene Therapy
8.4.5 Tissue and Tissue Products
8.4.6 Stem Cell Products
8.4.7 Others
Section-9 North America Biological Safety Testing Market Analysis
9.1 North America Market Overview
9.2 North America Market Value Share Analysis, By Product Type
9.2.1 North America Market Forecast By Product Type
9.3 North America Market Value Share Analysis, By Test
9.3.1 North America Market Forecast By Test
9.4 North America Market Value Share Analysis, By Application
9.4.1 North America Market Forecast By Application
9.5 North America Market Value Share Analysis, By Country
9.5.1 North America Market Value Share Analysis Forecast, by Country
9.6 Key Trends
Section-10 Europe Biological Safety Testing Market Analysis
10.1 Europe Market Overview
10.2 Europe Market Value Share Analysis, By Product Type
10.2.1 Europe Market Forecast by Product Type
10.3 Europe Market Value Share Analysis, By Test
10.3.1 Europe Market Forecast by Test
10.4 Europe Market Value Share Analysis, By Application
10.4.1 Europe Market Forecast by Application
10.5 Europe Market Value Share Analysis, By Country
10.5.1 Europe Market Value Share Analysis Forecast, by Country
10.6 Key Trends
Section-11 Asia Pacific Biological Safety Testing Market Analysis
11.1 Asia Pacific Market Overview
11.2 Asia Pacific Market Value Share Analysis, By Product Type
11.2.1 Asia Pacific Market Forecast by Product Type
11.3 Asia Pacific Market Value Share Analysis, By Test
11.3.1 Asia Pacific Market Forecast by Test
11.4 Asia Pacific Market Value Share Analysis, By Application
11.4.1 Asia Pacific Market Forecast by Application
11.5 Asia Pacific Market Value Share Analysis, By Country
11.5.1 Asia Pacific Market Value Share Analysis Forecast, by Country
11.6 Key Trends
Section-12 Latin America Biological Safety Testing Market Analysis
12.1 Latin America Market Overview
12.2 Latin America Market Value Share Analysis, By Product Type
12.2.1 Latin America Market Forecast by Product Type
12.3 Latin America Market Value Share Analysis, By Test
12.3.1 Latin America Market Forecast by Test
12.4 Latin America Market Value Share Analysis, By Application
12.4.1 Latin America Market Forecast by Application
12.5 Latin America Market Value Share Analysis, By Country
12.5.1 Latin America Market Value Share Analysis Forecast, by Country
12.6 Key Trends
Section-13 Middle East & Africa Biological Safety Testing Market Analysis
13.1 Middle East & Africa Market Overview
13.2 Middle East & Africa Market Value Share Analysis, By Product Type
13.2.1 Middle East & Africa Market Forecast by Product Type
13.3 Middle East & Africa Market Value Share Analysis, By Test
13.3.1 Middle East & Africa Market Forecast by Test
13.4 Middle East & Africa Market Value Share Analysis, By Application
13.4.1 Middle East & Africa Market Forecast by Application
13.5 Middle East & Africa Market Value Share Analysis, By Country
13.5.1 Middle East & Africa Market Value Share Analysis Forecast, by Country
13.6 Key Trends
Section-14 Competition Landscape
14.1. Competition Matrix
14.2. Company Profiles
14.2.1. Avance Biosciences
14.2.1.1 Company Overview
14.2.1.2 Business Overview
14.2.1.3 Financial Overview
14.2.1.4 SWOT Analysis
14.2.1.5 Strategic Overview
14.2.2 Charles River Laboratories
14.2.2.1 Company Overview
14.2.2.2 Business Overview
14.2.2.3 Financial Overview
14.2.2.4 SWOT Analysis
14.2.2.5 Strategic Overview
14.2.3 Lonza Group
14.2.3.1 Company Overview
14.2.3.2 Business Overview
14.2.3.3 Financial Overview
14.2.3.4 SWOT Analysis
14.2.3.5 Strategic Overview
14.2.4 Merck KGaA
14.2.4.1 Company Overview
14.2.4.2 Business Overview
14.2.4.3 Financial Overview
14.2.4.4 SWOT Analysis
14.2.4.5 Strategic Overview
14.2.5 Nordic Scientific & Natural Solutions AB.
14.2.5.1 Company Overview
14.2.5.2 Business Overview
14.2.5.3 Financial Overview
14.2.5.4 SWOT Analysis
14.2.5.5 Strategic Overview
14.2.6 NuAire
14.2.6.1 Company Overview
14.2.6.2 Business Overview
14.2.6.3 Financial Overview
14.2.6.4 SWOT Analysis
14.2.6.5 Strategic Overview
14.2.7 Paragon Bioservices, Inc.
14.2.7.1 Company Overview
14.2.7.2 Business Overview
14.2.7.3 Financial Overview
14.2.7.4 SWOT Analysis
14.2.7.5 Strategic Overview
14.2.8 SGS S.A.
14.2.8.1 Company Overview
14.2.8.2 Business Overview
14.2.8.3 Financial Overview
14.2.8.4 SWOT Analysis
14.2.8.5 Strategic Overview
14.2.9 Thermo Fisher Scientific Inc.
14.2.9.1 Company Overview
14.2.9.2 Business Overview
14.2.9.3 Financial Overview
14.2.9.4 SWOT Analysis
14.2.9.5 Strategic Overview
14.2.10. Toxicon Corporation
14.2.10.1 Company Overview
14.2.10.2 Business Overview
14.2.10.3 Financial Overview
14.2.10.4 SWOT Analysis
14.2.10.5 Strategic Overview
14.2.11. Wuxi Pharmatech (Cayman) Inc.
14.2.11.1 Company Overview
14.2.11.2 Business Overview
14.2.11.3 Financial Overview
14.2.11.4 SWOT Analysis
14.2.11.5 Strategic Overview
List of Tables
Table 1: Global Biological Safety Testing Market Size (US$ Mn) Forecast, by Product Type, 2014–2024
Table 2: Global Biological Safety Testing Market Size (US$ Mn) Forecast, by Instrument Type, 2014–2024
Table 3: Global Biological Safety Testing Market Size (US$ Mn) Forecast, by Test Type, 2014–2024
Table 4: Global Biological Safety Testing Market Size (US$ Mn) Forecast, by Application, 2014–2024
Table 5: Global Biological Safety Testing Market Size (US$ Mn) Forecast, by Region, 2014–2024
Table 6: North America Biological Safety Testing Market Size (US$ Mn) Forecast, by Product Type, 2014–2024
Table 7: North America Biological Safety Testing Market Size (US$ Mn) Forecast, by Instrument Type, 2014–2024
Table 8: North America Biological Safety Testing Market Size (US$ Mn) Forecast, by Test Type, 2014–2024
Table 9: North America Biological Safety Testing Market Size (US$ Mn) Forecast, by Application, 2014–2024
Table 10: North America Biological Safety Testing Market Size (US$ Mn) Forecast, by Country, 2014–2024
Table 11: Europe Biological Safety Testing Market Size (US$ Mn) Forecast, by Product Type, 2014–2024
Table 12: Europe Biological Safety Testing Market Size (US$ Mn) Forecast, by Instrument Type, 2014–2024
Table 13: Europe Biological Safety Testing Market Size (US$ Mn) Forecast, by Test Type, 2014–2024
Table 14: Europe Biological Safety Testing Market Size (US$ Mn) Forecast, by Application, 2014–2024
Table 15: Europe Biological Safety Testing Market Size (US$ Mn) Forecast, by Country, 2014–2024
Table 16: Asia Pacific Biological Safety Testing Market Size (US$ Mn) Forecast, by Product Type, 2014–2024
Table 17: Asia Pacific Biological Safety Testing Market Size (US$ Mn) Forecast, by Instrument Type, 2014–2024
Table 18: Asia Pacific Biological Safety Testing Market Size (US$ Mn) Forecast, by Test Type, 2014–2024
Table 19: Asia Pacific Biological Safety Testing Market Size (US$ Mn) Forecast, by Application, 2014–2024
Table 20: Asia Pacific Biological Safety Testing Market Size (US$ Mn) Forecast, by Country, 2014–2024
Table 21: Latin America Biological Safety Testing Market Size (US$ Mn) Forecast, by Product Type, 2014–2024
Table 22: Latin America Biological Safety Testing Market Size (US$ Mn) Forecast, by Instrument Type, 2014–2024
Table 23: Latin America Biological Safety Testing Market Size (US$ Mn) Forecast, by Test Type, 2014–2024
Table 24: Latin America Biological Safety Testing Market Size (US$ Mn) Forecast, by Application Type, 2014–2024
Table 25: Latin America Biological Safety Testing Market Size (US$ Mn) Forecast, by Country, 2014–2024
Table 26: Middle East & Africa Biological Safety Testing Market Size (US$ Mn) Forecast, by Product Type, 2014–2024
Table 27: Middle East & Africa Biological Safety Testing Market Size (US$ Mn) Forecast, by Instrument Type, 2014–2024
Table 28: Middle East & Africa Biological Safety Testing Market Size (US$ Mn) Forecast, by Test Type, 2014–2024
Table 29: Middle East & Africa Biological Safety Testing Market Size (US$ Mn) Forecast, by Application, 2014–2024
Table 30: Middle East & Africa Biological Safety Testing Market Size (US$ Mn) Forecast, by Country, 2014–2024
List of Figures
Figure 1: Global Biological Safety Testing Market Size (US$ Mn) Forecast, 2014–2024
Figure 2: Global Biological Safety Testing Market Value Share Analysis, by Product Type, 2016 and 2024
Figure 3: Global Instruments Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014– 2024
Figure 4: Global Reagents & Kits Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 5: Biological Safety Testing Market Attractiveness Analysis,
Figure 6: Global Biological Safety Testing Market Value Share Analysis, by Test Type, 2016 and 2024
Figure 7: Global Endotoxin Tests Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 8: Global Sterility Tests Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014– 2024
Figure 9: Global Cell Line Authentication and Characterization Tests Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 10: Global Bioburden Tests Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 11: Global Residual Host Contaminant Detection Tests Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 12: Global Adventitious Agent Detection Tests Market Revenue (US$ Mn) and Y- o-Y Growth (%), 2014–2024
Figure 13: Global Other Tests Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014– 2024
Figure 14: Biological Safety Testing Market Attractiveness Analysis, by Test Type
Figure 15: Global Biological Safety Testing Market Value Share Analysis, by Technology, 2016 and 2024
Figure 16: Global Vaccines & Therapeutics Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 17: Global Blood & Blood Products Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 18: Global Tissue & Tissue Products Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 19: Global Stem Cell Products Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014– 2024
Figure 20: Global Gene Therapy Products Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 21: Global Cellular Therapy Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2024
Figure 22: Biological Safety Testing Market Attractiveness Analysis, by Application
Figure 23: Global Biological Safety Testing Market Value Share Analysis, by Region, 2016 and 2024
Figure 24: Biological Safety Testing Market Attractiveness Analysis, by Region
Figure 25: North America Biological Safety Testing Market Size (US$ Mn) Forecast, 2016–2024
Figure 26: North America Biological Safety Testing Market Size, 2015–2024
Figure 27: North America Biological Safety Testing Market Attractiveness Analysis, by Country
Figure 28: North America Biological Safety Testing Market Value Share Analysis, by Product Type, 2016 and 2024
Figure 29: Biological Safety Testing Market Attractiveness Analysis, by Product Type
Figure 30: North America Biological Safety Testing Market Value Share Analysis, by Test Type, 2016 and 2024
Figure 31: Biological Safety Testing Market Attractiveness Analysis, by Test Type
Figure 32: North America Biological Safety Testing Market Value Share Analysis, by Application, 2016 and 2024
Figure 33: Biological Safety Testing Market Attractiveness Analysis, by Application
Figure 34: North America Market Value Share Analysis, by Country, 2016 and 2024
Figure 35: Europe Biological Safety Testing Market Size (US$ Mn) Forecast, 2016–2024
Figure 36: Europe Biological Safety Testing Market Size, 2015–2024
Figure 37: Europe Biological Safety Testing Market Attractiveness Analysis, by Country
Figure 38: Europe Biological Safety Testing Market Value Share Analysis, by Product Type, 2016 and 2024
Figure 39: Biological Safety Testing Market Attractiveness Analysis, by Product Type
Figure 40: Europe Biological Safety Testing Market Value Share Analysis, by Test Type, 2016 and 2024
Figure 41: Biological Safety Testing Market Attractiveness Analysis, by Test Type
Figure 42: Europe Biological Safety Testing Market Value Share Analysis, by Application, 2016 and 2024
Figure 43: Biological Safety Testing Market Attractiveness Analysis, by Application
Figure 44: Europe Market Value Share Analysis, by Country, 2016 and 2024
Figure 45: Asia Pacific Biological Safety Testing Market Size (US$ Mn) Forecast, 2016–2024
Figure 46: Asia Pacific Biological Safety Testing Market Size, 2015–2024
Figure 47: Asia Pacific Biological Safety Testing Market Attractiveness Analysis, by Country
Figure 48: Asia Pacific Biological Safety Testing Market Value Share Analysis, by Product Type, 2016 and 2024
Figure 49: Biological Safety Testing Market Attractiveness Analysis, by Product Type
Figure 50: Asia Pacific Biological Safety Testing Market Value Share Analysis, by Test Type, 2016 and 2024
Figure 51: Biological Safety Testing Market Attractiveness Analysis, by Test Type
Figure 52: Asia Pacific Biological Safety Testing Market Value Share Analysis, by Application, 2016 and 2024
Figure 53: Biological Safety Testing Market Attractiveness Analysis, by Application
Figure 54: Asia Pacific Market Value Share Analysis, by Country, 2016 and 2024
Figure 55: Latin America Biological Safety Testing Market Size (US$ Mn) Forecast,
Figure 56: Latin America Biological Safety Testing Market Y-o-Y Growth (%) Projection, 2015– 2024
Figure 57: Latin America Biological Safety Testing Market Attractiveness Analysis, by Country
Figure 58: Latin America Biological Safety Testing Market Value Share Analysis, by Product Type, 2016 and 2024
Figure 59: Latin America Biological Safety Testing Market Attractiveness Analysis, by Product Type, 2016–2024
Figure 60: Latin America Biological Safety Testing Market Value Share Analysis, by Test Type, 2016 and 2024
Figure 61: Latin America Biological Safety Testing Market Attractiveness Analysis, by Test Type, 2016–2024
Figure 62: Latin America Biological Safety Testing Market Value Share Analysis, by Application Type, 2016 and 2024
Figure 63: Latin America Biological Safety Testing Market Attractiveness Analysis, by Application Type, 2016–2024
Figure 64: Latin America Biological Safety Testing Market Value Share Analysis, by Country, 2016 and 2024
Figure 65: Middle East & Africa Biological Safety Testing Market Size (US$ Mn) Forecast, 2016– 2024
Figure 66: Middle East & Africa Biological Safety Testing Market Size, 2015–2024
Figure 67: Middle East & Africa Biological Safety Testing Market Attractiveness Analysis, by Country
Figure 68: Middle East & Africa Biological Safety Testing Market Value Share Analysis, by Product Type, 2016 and 2024
Figure 69: Biological Safety Testing Market Attractiveness Analysis, by Product Type
Figure 70: Middle East & Africa Biological Safety Testing Market Value Share Analysis, by Test Type, 2016 and 2024
Figure 71: Biological Safety Testing Market Attractiveness Analysis, by Test Type
Figure 72: Middle East & Africa Biological Safety Testing Market Value Share Analysis, by Application, 2016 and 2024
Figure 73: Biological Safety Testing Market Attractiveness Analysis, by Application
Figure 74: Middle East & Africa Market Value Share Analysis, by Country, 2016 and 2024
Figure 75: Global Biological Safety Testing Market Share Analysis, by Company (2015)