Reports
Reports
Analysts’ Viewpoint
Increase in incidence of atrial fibrillation and rise in demand for minimally invasive procedures are driving the global atrial fibrillation surgery devices market. Rise in awareness among patients and physicians about the benefits of using atrial fibrillation surgery devices is expected to propel market expansion. Furthermore, technological advancements, such as the development of advanced catheters, ablation devices, and mapping systems that are expected to provide better outcomes and improve patient safety, are accelerating market development.
Growing trend of personalized medicine and increase in focus on patient-centric healthcare offer lucrative opportunities to companies in the market. Manufacturers are focusing development of innovative and advanced atrial fibrillation surgery devices to increase market share. However, high cost of devices and shortage of skilled personnel to perform the procedures are likely to hamper market growth in the near future.
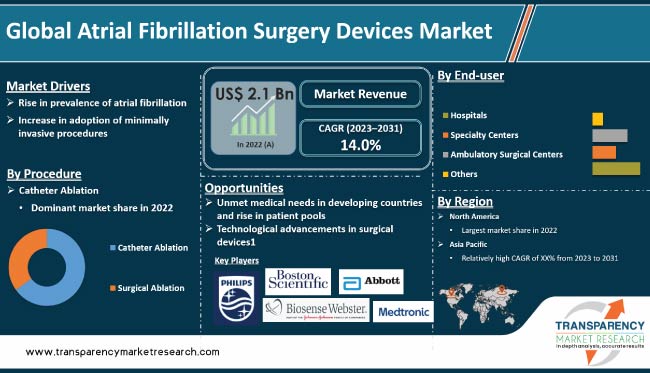
Atrial fibrillation (AF) surgery devices are designed to treat atrial fibrillation, a common heart arrhythmia that causes irregular and often rapid heartbeats. These devices work by either ablating or blocking electrical signals in the heart that cause the arrhythmia. Examples of AF surgery devices are catheter-based ablation systems, surgical ablation devices, and left atrial appendage closure devices. These devices are used in conjunction with other treatments, such as medications or lifestyle changes, to manage AF and reduce the risk of stroke or other complications.
Increase in prevalence of AF across the world is driving the global atrial fibrillation surgery devices market share. It is one of the most common arrhythmias, and affects millions of people globally. AF is associated with increased risk of stroke, heart failure, and other cardiovascular events. Surge in the geriatric population is increasing the prevalence of AF. Risk of developing AF increases as people get older. According to a study published in the Journal of the American College of Cardiology, prevalence of AF increases from less than 0.5% in people under 50 to more than 10% in people aged over 80.
Changes in lifestyle also increases the prevalence of AF. For example, obesity, diabetes, and high blood pressure are the major risk factors for AF. These conditions have become more common in the past few years, which has contributed to the rise in prevalence of AF. According to the World Health Organization, the global prevalence of obesity nearly tripled between 1975 and 2019. Increase in alcohol consumption, stress, and sleep apnea are the other factors contributing to rise in prevalence of AF. Alcohol consumption has been linked to increased risk of AF, and studies have suggested that stress and sleep apnea could also be risk factors for the condition.
Adoption of minimally invasive procedures has increased in the past few years. Minimally invasive procedures offer several advantages over traditional open surgeries, including shorter hospital stay, reduced pain & scarring, and faster recovery. This has led to increase in demand for minimally invasive procedures, including those used to treat atrial fibrillation (AF).
Catheter ablation is one of the primary minimally invasive procedures used to treat AF. It is a procedure in which a thin, flexible tube called a catheter is inserted into a blood vessel in the groin and threaded up to the heart. Once the catheter is in place, it delivers energy to the tissue responsible for abnormal heartbeats, destroying or isolating it and restoring normal heart rhythm. Catheter ablation has several advantages over traditional open surgical approaches to treating AF. Additionally, catheter ablation has been shown to be more effective in restoring normal heart rhythm than medication alone.
Adoption of catheter ablation has increased in the past few years. According to a study published in the Journal of the American College of Cardiology, usage of catheter ablation for the treatment of AF increased by more than 700% between 2000 and 2018. This trend is expected to continue, as increasing number of healthcare providers adopt this minimally invasive approach to treat AF. These factors are likely to bolster global atrial fibrillation surgery devices market growth in the next few years.
In terms of procedure, the catheter ablation segment accounted for largest share of 70.0% of the global atrial fibrillation surgery devices market size in 2022. Increase in prevalence of cardiovascular diseases is likely to drive the segment in the next few years. The catheter ablation segment has been bifurcated into radiofrequency ablation and cryoablation. Radiofrequency is currently the most commonly used energy source in catheter ablation procedures. The procedure involves inserting a catheter into the heart through a vein in the groin or neck and using radiofrequency energy to destroy the abnormal tissue.
Based on end-user, the hospitals segment is likely to account for leading share of the global atrial fibrillation surgery devices market during the forecast period. Hospitals are the preferred settings for atrial fibrillation surgeries, as these have the required equipment and personnel to perform the procedure. Atrial fibrillation surgeries could also be performed in ambulatory surgery centers and other specialized facilities.
North America accounted for around 35.0% of the global atrial fibrillation surgery devices market demand in 2022. The region is expected to be a highly lucrative market during the forecast period. North America's global dominance is ascribed to increase in incidence cardiac arrhythmia. The U.S. dominated the market in the region owing to presence of leading players and increase in research & development activities in atrial fibrillation surgery devices.
Asia Pacific is projected to be the fastest growing market for atrial fibrillation surgery devices during the forecast period. The market in the region is anticipated to be driven by high incidence rate of AF and growing population.
The global atrial fibrillation surgery devices market is fragmented, with the presence of large number of key players. Leading companies are investing significantly in research & development, primarily to develop innovative products. Expansion of product portfolio and merger & acquisition are the key strategies adopted by the leading players in the market. Abbott Laboratories., Biosense Webster, Inc., Medtronic plc, Koninklijke Philips N.V., Boston Scientific Corporation, CardioFocus, Inc., AtriCure, Inc., Osypka AG, Kardium, Inc., and Acutus Medical, Inc. are the prominent players operating in the market.
Each of these players has been profiled in the atrial fibrillation surgery devices market report based on parameters such as company overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Size in 2021 |
US$ 2.1 Bn |
|
Forecast (Value) in 2031 |
US$ 7.0 Bn |
|
Growth Rate (CAGR) |
14.0% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2020 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Furthermore, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global industry was valued at US$ 2.1 Bn in 2022
It is projected to reach more than US$ 7.0 Bn by 2031
The CAGR is anticipated to be 14.0% from 2023 to 2031
Rise prevalence of atrial fibrillation, increase in adoption of minimally invasive procedures, surge in the geriatric population, and changing lifestyle leading to cardiovascular diseases are expected to drive the global market.
The catheter ablation segment held over 60.0% share in 2022
North America is expected to capture leading share during the forecast period.
Abbott Laboratories, Biosense Webster, Inc., Medtronic plc, Boston Scientific Corporation, CardioFocus, Inc., AtriCure, Inc., Osypka AG, Kardium, Inc., Acutus Medical, Inc., and Koninklijke Philips N.V. are the prominent players in the global atrial fibrillation surgery devices market.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Atrial Fibrillation Surgery Devices Market
4. Market Overview
4.1. Introduction
4.1.1. Procedure Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Atrial Fibrillation Surgery Devices Market Analysis and Forecast, 2017–2031
4.4.1. Porter’s Five Forces Analysis
5. Key Insights
5.1. Disease Prevalence & Incidence Rate Globally With Key Countries
5.2. Technological Advancements
5.3. Overview of Atrial Fibrillation
5.4. COVID-19 Pandemic Impact on Industry (value chain and short/mid/long term impact)
6. Global Atrial Fibrillation Surgery Devices Market Analysis and Forecast, by Procedure
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Procedure, 2017–2031
6.3.1. Catheter Ablation
6.3.1.1. Radiofrequency Ablation
6.3.1.2. Cryoablation
6.3.2. Surgical Ablation
6.3.2.1. Convergent Ablation
6.3.2.2. Maze Ablation
6.4. Market Attractiveness Analysis, by Procedure
7. Global Atrial Fibrillation Surgery Devices Market Analysis and Forecast, by End-user
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by End-user, 2017–2031
7.3.1. Hospitals
7.3.2. Specialty Centers
7.3.3. Ambulatory Surgical Centers
7.3.4. Others
7.4. Market Attractiveness Analysis, by End-user
8. Global Atrial Fibrillation Surgery Devices Market Analysis and Forecast, by Region
8.1. Key Findings
8.2. Market Value Forecast, by Region
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Latin America
8.2.5. Middle East & Africa
8.3. Market Attractiveness Analysis, by Region
9. North America Atrial Fibrillation Surgery Devices Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value Forecast, by Procedure, 2017–2031
9.2.1. Catheter Ablation
9.2.1.1. Radiofrequency Ablation
9.2.1.2. Cryoablation
9.2.2. Surgical Ablation
9.2.2.1. Convergent Ablation
9.2.2.2. Maze Ablation
9.3. Market Value Forecast, by End-user, 2017–2031
9.3.1. Hospitals
9.3.2. Specialty Centers
9.3.3. Ambulatory Surgical Centers
9.3.4. Others
9.4. Market Value Forecast, by Country, 2017–2031
9.4.1. U.S.
9.4.2. Canada
9.5. Market Attractiveness Analysis
9.5.1. By Procedure
9.5.2. By End-user
9.5.3. By Country
10. Europe Atrial Fibrillation Surgery Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Procedure, 2017–2031
10.2.1. Catheter Ablation
10.2.1.1. Radiofrequency Ablation
10.2.1.2. Cryoablation
10.2.2. Surgical Ablation
10.2.2.1. Convergent Ablation
10.2.2.2. Maze Ablation
10.3. Market Value Forecast, by End-user, 2017–2031
10.3.1. Hospitals
10.3.2. Specialty Centers
10.3.3. Ambulatory Surgical Centers
10.3.4. Others
10.4. Market Value Forecast, by Country/Sub-region, 2017–2031
10.4.1. Germany
10.4.2. U.K.
10.4.3. France
10.4.4. Italy
10.4.5. Spain
10.4.6. Rest of Europe
10.5. Market Attractiveness Analysis
10.5.1. By Procedure
10.5.2. By End-user
10.5.3. By Country/Sub-region
11. Asia Pacific Atrial Fibrillation Surgery Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Procedure, 2017–2031
11.2.1. Catheter Ablation
11.2.1.1. Radiofrequency Ablation
11.2.1.2. Cryoablation
11.2.2. Surgical Ablation
11.2.2.1. Convergent Ablation
11.2.2.2. Maze Ablation
11.3. Market Value Forecast, by End-user, 2017–2031
11.3.1. Hospitals
11.3.2. Specialty Centers
11.3.3. Ambulatory Surgical Centers
11.3.4. Others
11.4. Market Value Forecast, by Country/Sub-region, 2017–2031
11.4.1. China
11.4.2. Japan
11.4.3. India
11.4.4. Australia & New Zealand
11.4.5. Rest of Asia Pacific
11.5. Market Attractiveness Analysis
11.5.1. By Procedure
11.5.2. By End-user
11.5.3. By Country/Sub-region
12. Latin America Atrial Fibrillation Surgery Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Procedure, 2017–2031
12.2.1. Catheter Ablation
12.2.1.1. Radiofrequency Ablation
12.2.1.2. Cryoablation
12.2.2. Surgical Ablation
12.2.2.1. Convergent Ablation
12.2.2.2. Maze Ablation
12.3. Market Value Forecast, by End-user, 2017–2031
12.3.1. Hospitals
12.3.2. Specialty Centers
12.3.3. Ambulatory Surgical Centers
12.3.4. Others
12.4. Market Value Forecast, by Country/Sub-region, 2017–2031
12.4.1. Brazil
12.4.2. Mexico
12.4.3. Rest of Latin America
12.5. Market Attractiveness Analysis
12.5.1. By Procedure
12.5.2. By End-user
12.5.3. By Country/Sub-region
13. Middle East & Africa Atrial Fibrillation Surgery Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Procedure, 2017–2031
13.2.1. Catheter Ablation
13.2.1.1. Radiofrequency Ablation
13.2.1.2. Cryoablation
13.2.2. Surgical Ablation
13.2.2.1. Convergent Ablation
13.2.2.2. Maze Ablation
13.3. Market Value Forecast, by End-user, 2017–2031
13.3.1. Hospitals
13.3.2. Specialty Centers
13.3.3. Ambulatory Surgical Centers
13.3.4. Others
13.4. Market Value Forecast, by Country/Sub-region, 2017–2031
13.4.1. GCC Countries
13.4.2. South Africa
13.4.3. Rest of Middle East & Africa
13.5. Market Attractiveness Analysis
13.5.1. By Procedure
13.5.2. By End-user
13.5.3. By Country/Sub-region
14. Competition Landscape
14.1. Market Player - Competition Matrix (by tier and size of companies)
14.2. Market Share Analysis, by Company (2022)
14.3. Company Profiles
14.3.1. AtriCure, Inc.
14.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.1.2. Product Portfolio
14.3.1.3. Financial Overview
14.3.1.4. SWOT Analysis
14.3.1.5. Strategic Overview
14.3.2. CardioFocus, Inc.
14.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.2.2. Product Portfolio
14.3.2.3. Financial Overview
14.3.2.4. SWOT Analysis
14.3.2.5. Strategic Overview
14.3.3. Abbott Laboratories
14.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.3.2. Product Portfolio
14.3.3.3. Financial Overview
14.3.3.4. SWOT Analysis
14.3.3.5. Strategic Overview
14.3.4. Biosense Webster, Inc.
14.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.4.2. Product Portfolio
14.3.4.3. Financial Overview
14.3.4.4. SWOT Analysis
14.3.4.5. Strategic Overview
14.3.5. Medtronic plc
14.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.5.2. Product Portfolio
14.3.5.3. Financial Overview
14.3.5.4. SWOT Analysis
14.3.6. Boston Scientific Corporation
14.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.6.2. Product Portfolio
14.3.6.3. Financial Overview
14.3.6.4. SWOT Analysis
14.3.7. Osypka AG
14.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.7.2. Product Portfolio
14.3.7.3. Financial Overview
14.3.7.4. SWOT Analysis
14.3.8. Kardium, Inc.
14.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.8.2. Product Portfolio
14.3.8.3. Financial Overview
14.3.8.4. SWOT Analysis
14.3.9. Acutus Medical, Inc.
14.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.9.2. Product Portfolio
14.3.9.3. Financial Overview
14.3.9.4. SWOT Analysis
14.3.10. Koninklijke Philips N.V.
14.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.10.2. Product Portfolio
14.3.10.3. Financial Overview
14.3.10.4. SWOT Analysis
14.3.10.5. Strategic Overview
List of Tables
Table 01: Global Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Procedure, 2017–2031
Table 02: Global Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 03: Global Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 04: North America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Procedure, 2017–2031
Table 05: North America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 06: North America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 07: Europe Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Procedure, 2017–2031
Table 08: Europe Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 09: Europe Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 10: Asia Pacific Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Procedure, 2017–2031
Table 11: Asia Pacific Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 12: Asia Pacific Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 13: Latin America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Procedure, 2017–2031
Table 14: Latin America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 15: Latin America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 16: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Procedure, 2017–2031
Table 17: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 18: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
List of Figures
Figure 01: Global Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Global Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Procedure, 2022 and 2031
Figure 03: Global Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Procedure, 2023–2031
Figure 04: Global Atrial Fibrillation Surgery Devices Market Value Share Analysis, by End-user, 2022 and 2031
Figure 05: Global Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by End-user, 2023–2031
Figure 06: Global Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Region, 2022 and 2031
Figure 07: Global Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Region, 2023–2031
Figure 08: North America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 09: North America Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Procedure, 2022 and 2031
Figure 10: North America Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Procedure, 2023–2031
Figure 11: North America Atrial Fibrillation Surgery Devices Market Value Share Analysis, by End-user, 2022 and 2031
Figure 12: North America Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by End-user, 2023–2031
Figure 13: North America Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Country, 2022 and 2031
Figure 14: North America Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Country, 2023–2031
Figure 15: Europe Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 16: Europe Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Procedure, 2022 and 2031
Figure 17: Europe Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Procedure, 2023–2031
Figure 18: Europe Atrial Fibrillation Surgery Devices Market Value Share Analysis, by End-user, 2022 and 2031
Figure 19: Europe Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by End-user, 2023–2031
Figure 20: Europe Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 21: Europe Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 22: Asia Pacific Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 23: Asia Pacific Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Procedure, 2022 and 2031
Figure 24: Asia Pacific Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Procedure, 2023–2031
Figure 25: Asia Pacific Atrial Fibrillation Surgery Devices Market Value Share Analysis, by End-user, 2022 and 2031
Figure 26: Asia Pacific Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by End-user, 2023–2031
Figure 27: Asia Pacific Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 28: Asia Pacific Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 29: Latin America Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 30: Latin America Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Procedure, 2022 and 2031
Figure 31: Latin America Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Procedure, 2023–2031
Figure 32: Latin America Atrial Fibrillation Surgery Devices Market Value Share Analysis, by End-user, 2022 and 2031
Figure 33: Latin America Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by End-user, 2023–2031
Figure 34: Latin America Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 35: Latin America Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 36: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value (US$ Mn) Forecast, 2017–2031
Figure 37: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Procedure, 2022 and 2031
Figure 38: Middle East & Africa Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Procedure, 2023–2031
Figure 39: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value Share Analysis, by End-user, 2022 and 2031
Figure 40: Middle East & Africa Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by End-user, 2023–2031
Figure 41: Middle East & Africa Atrial Fibrillation Surgery Devices Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 42: Middle East & Africa Atrial Fibrillation Surgery Devices Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 43: Global Atrial Fibrillation Surgery Devices Market Share Analysis, by Company, 2022