Reports
Reports
Analysts’ Viewpoint
Rise in prevalence of amyotrophic lateral sclerosis is driving the global amyotrophic lateral sclerosis (ALS) treatment market. Sporadic ALS is the most common type of ALS, which affects around 90% to 95% of total ALS patients. Increase in awareness among patients and healthcare professionals about new treatment options is expected to propel global amyotrophic lateral sclerosis (ALS) treatment market growth. Emergence of stem cell therapy as a new treatment option for ALS is likely to bolster market expansion.
The COVID-19 pandemic had a negative effect on the overall amyotrophic lateral sclerosis (ALS) treatment market share. Moreover, high cost associated with amyotrophic lateral sclerosis (ALS) treatment is projected to hamper market growth in the near future.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a progressive and debilitating neurodegenerative disorder that affects the nerve cells responsible for controlling voluntary muscle movement. It was first described by French neurologist Jean-Martin Charcot in the late 19th century.
ALS primarily affects the motor neurons, which are the nerve cells responsible for transmitting signals from the brain and spinal cord to the muscles throughout the body. As the disease progresses, these motor neurons gradually degenerate and die, leading to a loss of muscle control and function.
Amyotrophic lateral sclerosis symptoms typically manifest as muscle weakness, muscle wasting, and a progressive decline in voluntary muscle control. Initial signs could include difficulty with tasks that require fine motor skills, such as buttoning a shirt or writing. As the disease progresses, individuals could experience difficulty walking, speaking, swallowing, and eventually breathing. However, cognitive function is typically preserved in most cases of ALS.
The course of ALS can vary from person to person, but it generally follows a relentless and progressive course, leading to severe disability and ultimately respiratory failure, which is the most common cause of death in ALS patients.
The average amyotrophic lateral sclerosis life expectancy after diagnosis is around two to five years, although some individuals may live longer with appropriate medical care and support. Amyotrophic lateral sclerosis supportive therapy includes physiotherapy, which works to maintain physical function. Occupational therapy helps find strategies for navigating daily life with a chronic condition. Speech therapy helps with speech problems as well as difficulty swallowing.
Increase in prevalence of amyotrophic lateral sclerosis is a major factor projected to drive the global amyotrophic lateral sclerosis treatment market size during the forecast period. Prevalence of ALS has been steadily increasing; an estimated two to five per 100,000 people are affected by ALS worldwide. Here are some factors that contribute to the growing prevalence of ALS:
A robust product pipeline means there are multiple potential treatments in various stages of development, including preclinical studies, clinical trials, and regulatory review. This diversification of treatment options provides hope for patients and physicians, as it increases the likelihood of finding effective therapies to manage ALS.
NurOwn, developed by BrainStorm Cell Therapeutics, is an investigational therapy that utilizes mesenchymal stem cells (MSCs) derived from the patient's bone marrow. These MSCs are modified to secrete factors that support the survival and function of motor neurons. NurOwn has completed phase 3 clinical trials, and results have shown promising outcomes in terms of slowing ALS progression and improving patient function.
AMX0035 is a lead pipeline product by Amylyx Pharmaceuticals. It is a combination therapy consisting of sodium phenylbutyrate and taurursodiol. It aims to protect against neurodegeneration and preserve the health of motor neurons. Phase 2 clinical trials demonstrated a significant slowing of ALS progression and improvement in survival rates.
Thus, strong product pipeline is likely to bolster the global amyotrophic lateral sclerosis treatment market development in the next few years.
In terms of type, the sporadic amyotrophic lateral sclerosis (ALS) segment accounted for the largest global amyotrophic lateral sclerosis (ALS) treatment market share in 2022. Sporadic amyotrophic lateral sclerosis (ALS), often referred to as sporadic ALS or simply ALS, is the most common form of the neurodegenerative disease. ALS is a progressive disorder that affects the nerve cells responsible for controlling voluntary muscle movement in the brain and spinal cord.
Sporadic ALS is the most common form of the disease and accounts for approximately 90% to 95% of all ALS cases. Sporadic ALS is the occurrence of ALS without any apparent family history or genetic predisposition.
It can affect individuals of any age, but it most commonly manifests in people between the ages of 40 and 60. The exact cause of sporadic ALS remains largely unknown, although researchers believe it may result from a complex interaction of genetic, environmental, and lifestyle factors.
Based on treatment, the medication segment dominated the global amyotrophic lateral sclerosis (ALS) treatment market demand in 2022. Amyotrophic lateral sclerosis medication includes Rilutek (riluzole) and Radicava (edaravone).
Riluzole is a medication that has shown effectiveness in slowing down the progression of sporadic ALS. It works by reducing the release of glutamate, a neurotransmitter that can contribute to motor neuron damage. Riluzole has demonstrated to modestly extend survival and delay the need for tracheostomy in some patients.
Edaravone is another ALS medication approved for sporadic ALS treatment. It is an antioxidant that aims to reduce oxidative stress, which is believed to contribute to motor neuron degeneration. Edaravone has been shown to slow functional decline in some patients and is typically administered intravenously.
Rise in prevalence of amyotrophic lateral sclerosis and high demand for amyotrophic lateral sclerosis medications are likely to drive the segment during forecast period.
Based on distribution channel, the hospital pharmacies segment is likely to account for major share of the global amyotrophic lateral sclerosis treatment market during the forecast period. Amyotrophic lateral sclerosis patients need regular hospital visits. The number of patients being treated at hospitals is increasing due to favorable reimbursement policies.
The segment is anticipated to grow at a rapid pace during the forecast period. This is ascribed to rise in awareness about amyotrophic lateral sclerosis and the trend of utilization of new & improved drugs by patients and physicians.
As per amyotrophic lateral sclerosis treatment market trends, North America is a major market for amyotrophic lateral sclerosis treatment, with the U.S. being one of the largest markets for these medications. High prevalence of amyotrophic lateral sclerosis, aging population, and increase in demand for innovative treatment options for the disease are driving the market in North America.
Presence of large number of pharmaceutical companies and high awareness about amyotrophic lateral sclerosis treatment are expected to propel the market in North America in the next few years.
Asia Pacific is a rapidly growing market for amyotrophic lateral sclerosis treatment, with countries such as China and India being major markets for amyotrophic lateral sclerosis (ALS) drugs. Additionally, the rapidly growing healthcare sector in the region is expected to augment the market in the coming years.
Expansion of product portfolio and mergers & acquisitions are key strategies adopted by prominent manufacturers in the market. Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd., BrainStorm Therapeutics, Biogen Inc., Corestem, AB Science, F. Hoffmann-La Roche AG, Biohaven Pharmaceutical, and Sun Pharmaceutical are the prominent players in the market.
The amyotrophic lateral sclerosis treatment market report profiles key players based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
|
Size in 2022 |
US$ 594.1 Mn |
|
Forecast (Value) in 2031 |
More than US$ 958.2 Mn |
|
Growth Rate (CAGR) |
5.5% |
|
Forecast Period |
2023-2031 |
|
Historical Data Available for |
2017-2021 |
|
Quantitative Units |
US$ Mn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Furthermore, qualitative analysis includes drivers, restraints, opportunities, Porter’s Five Forces analysis, key trends, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
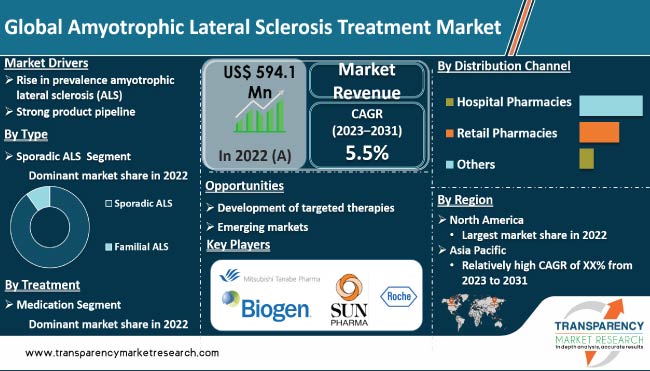
It was valued at US$ 594.1 Mn in 2022
It is projected to reach more than US$ 958.2 Mn by 2031
It is anticipated to grow at a CAGR of 5.5% from 2023 to 2031
Rise in prevalence amyotrophic lateral sclerosis (ALS) and strong product pipeline are propelling the demand for these medications.
North America is projected to be the most lucrative region during the forecast period
Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd., BrainStorm Therapeutics, Biogen, Inc., Corestem, AB Science, F. Hoffmann-La Roche AG, Biohaven Pharmaceutical, and Sun Pharmaceutical
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Amyotrophic Lateral Sclerosis Treatment Market
4. Market Overview
4.1. Introduction
4.1.1. Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast, 2017-2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Regulatory Scenario by Region/globally
5.2. Pipeline Analysis
5.3. Pricing Analysis
5.4. COVID-19 Pandemic Impact on Industry
6. Global Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast, by Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Type, 2017-2031
6.3.1. Sporadic ALS
6.3.2. Familial ALS
6.4. Market Attractiveness Analysis, by Type
7. Global Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast, by Treatment
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Treatment, 2017-2031
7.3.1. Medication
7.3.2. Stem Cell Therapy
7.3.3. Others
7.4. Market Attractiveness Analysis, by Treatment
8. Global Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast, by Distribution Channel
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Distribution Channel, 2017-2031
8.3.1. Hospital Pharmacies
8.3.2. Retail Pharmacies
8.3.3. Others
8.4. Market Attractiveness Analysis, by Distribution Channel
9. Global Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Type, 2017-2031
10.2.1. Sporadic ALS
10.2.2. Familial ALS
10.3. Market Value Forecast, by Treatment, 2017-2031
10.3.1. Medication
10.3.2. Stem Cell Therapy
10.3.3. Others
10.4. Market Value Forecast, by Distribution Channel, 2017-2031
10.4.1. Hospital Pharmacies
10.4.2. Retail Pharmacies
10.4.3. Others
10.5. Market Value Forecast, by Country, 2017-2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Type
10.6.2. By Treatment
10.6.3. By Distribution Channel
10.6.4. By Country
11. Europe Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Type, 2017-2031
11.2.1. Sporadic ALS
11.2.2. Familial ALS
11.3. Market Value Forecast, by Treatment, 2017-2031
11.3.1. Medication
11.3.2. Stem Cell Therapy
11.3.3. Others
11.4. Market Value Forecast, by Distribution Channel, 2017-2031
11.4.1. Hospital Pharmacies
11.4.2. Retail Pharmacies
11.4.3. Others
11.5. Market Value Forecast, by Country/Sub-region, 2017-2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Type
11.6.2. By Treatment
11.6.3. By Distribution Channel
11.6.4. By Country/Sub-region
12. Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Type, 2017-2031
12.2.1. Sporadic ALS
12.2.2. Familial ALS
12.3. Market Value Forecast, by Treatment, 2017-2031
12.3.1. Medication
12.3.2. Stem Cell Therapy
12.3.3. Others
12.4. Market Value Forecast, by Distribution Channel, 2017-2031
12.4.1. Hospital Pharmacies
12.4.2. Retail Pharmacies
12.4.3. Others
12.5. Market Value Forecast, by Country/Sub-region, 2017-2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Type
12.6.2. By Treatment
12.6.3. By Distribution Channel
12.6.4. By Country/Sub-region
13. Latin America Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Type, 2017-2031
13.2.1. Sporadic ALS
13.2.2. Familial ALS
13.3. Market Value Forecast, by Treatment, 2017-2031
13.3.1. Medication
13.3.2. Stem Cell Therapy
13.3.3. Others
13.4. Market Value Forecast, by Distribution Channel, 2017-2031
13.4.1. Hospital Pharmacies
13.4.2. Retail Pharmacies
13.4.3. Others
13.5. Market Value Forecast, by Country/Sub-region, 2017-2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Type
13.6.2. By Treatment
13.6.3. By Distribution Channel
13.6.4. By Country/Sub-region
14. Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Type, 2017-2031
14.2.1. Sporadic ALS
14.2.2. Familial ALS
14.3. Market Value Forecast, by Treatment, 2017-2031
14.3.1. Medication
14.3.2. Stem Cell Therapy
14.3.3. Others
14.4. Market Value Forecast, by Distribution Channel, 2017-2031
14.4.1. Hospital Pharmacies
14.4.2. Retail Pharmacies
14.4.3. Others
14.5. Market Value Forecast, by Country/Sub-region, 2017-2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Type
14.6.2. By Treatment
14.6.3. By Distribution Channel
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2022
15.3. Company Profiles
15.3.1. Mitsubishi Tanabe Pharma Corporation
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Financial Overview
15.3.1.4. SWOT Analysis
15.3.1.5. Strategic Overview
15.3.2. Otsuka Pharmaceutical Co., Ltd.
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. SWOT Analysis
15.3.2.5. Strategic Overview
15.3.3. BrainStorm Therapeutics
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Financial Overview
15.3.3.4. SWOT Analysis
15.3.3.5. Strategic Overview
15.3.4. Biogen, Inc.
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. SWOT Analysis
15.3.4.5. Strategic Overview
15.3.5. Corestem
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. SWOT Analysis
15.3.5.5. Strategic Overview
15.3.6. AB Science
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Financial Overview
15.3.6.4. SWOT Analysis
15.3.6.5. Strategic Overview
15.3.7. F. Hoffmann-La Roche AG
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. SWOT Analysis
15.3.7.5. Strategic Overview
15.3.8. Biohaven Pharmaceutical
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Financial Overview
15.3.8.4. SWOT Analysis
15.3.8.5. Strategic Overview
15.3.9. Sun Pharmaceutical
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. Financial Overview
15.3.9.4. SWOT Analysis
15.3.9.5. Strategic Overview
15.3.10. Other Prominent Players
List of Tables
Table 01: Global Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 02: Global Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Treatment, 2017-2031
Table 03: Global Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 04: Global Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Region, 2017-2031
Table 05: North America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 06: North America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Treatment, 2017-2031
Table 07: North America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 08: North America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Country, 2017-2031
Table 09: Europe Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 10: Europe Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Treatment, 2017-2031
Table 11: Europe Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 12: Europe Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 13: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 14: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Treatment, 2017-2031
Table 15: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 16: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 17: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 18: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Treatment, 2017-2031
Table 19: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 20: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 21: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Type, 2017-2031
Table 22: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Treatment, 2017-2031
Table 23: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Distribution Channel, 2017-2031
Table 24: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
List of Figures
Figure 01: Global Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, 2017-2031
Figure 02: Global Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Type, 2022 and 2031
Figure 03: Global Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Type, 2023-2031
Figure 04: Global Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Treatment, 2022 and 2031
Figure 05: Global Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Treatment, 2023-2031
Figure 06: Global Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Distribution Channel, 2022 and 2031
Figure 07: Global Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Distribution Channel, 2023-2031
Figure 08: Global Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Region, 2022 and 2031
Figure 09: Global Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Region, 2023-2031
Figure 10: North America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, 2017-2031
Figure 11: North America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Type, 2022 and 2031
Figure 12: North America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Type, 2023-2031
Figure 13: North America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Treatment, 2022 and 2031
Figure 14: North America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Treatment, 2023-2031
Figure 15: North America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Distribution Channel, 2022 and 2031
Figure 16: North America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Distribution Channel, 2023-2031
Figure 17: North America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Country, 2022 and 2031
Figure 18: North America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Country, 2023-2031
Figure 19: Europe Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, 2017-2031
Figure 20: Europe Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Type, 2022 and 2031
Figure 21: Europe Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Type, 2023-2031
Figure 22: Europe Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Treatment, 2022 and 2031
Figure 23: Europe Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Treatment, 2023-2031
Figure 24: Europe Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Distribution Channel, 2022 and 2031
Figure 25: Europe Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Distribution Channel, 2023-2031
Figure 26: Europe Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 27: Europe Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 28: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, 2017-2031
Figure 29: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Type, 2022 and 2031
Figure 30: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Type, 2023-2031
Figure 31: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Treatment, 2022 and 2031
Figure 32: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Treatment, 2023-2031
Figure 33: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Distribution Channel, 2022 and 2031
Figure 34: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Distribution Channel, 2023-2031
Figure 35: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 36: Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 37: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, 2017-2031
Figure 38: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Type, 2022 and 2031
Figure 39: Latin America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Type, 2023-2031
Figure 40: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Treatment, 2022 and 2031
Figure 41: Latin America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Treatment, 2023-2031
Figure 42: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Distribution Channel, 2022 and 2031
Figure 43: Latin America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Distribution Channel, 2023-2031
Figure 44: Latin America Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 45: Latin America Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 46: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value (US$ Mn) Forecast, 2017-2031
Figure 47: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Type, 2022 and 2031
Figure 48: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Type, 2023-2031
Figure 49: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Treatment, 2022 and 2031
Figure 50: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Treatment, 2023-2031
Figure 51: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Distribution Channel, 2022 and 2031
Figure 52: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Distribution Channel, 2023-2031
Figure 53: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 54: Middle East & Africa Amyotrophic Lateral Sclerosis Treatment Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 55: Global Amyotrophic Lateral Sclerosis Treatment Market Share Analysis, by Company, 2022