Reports
Reports
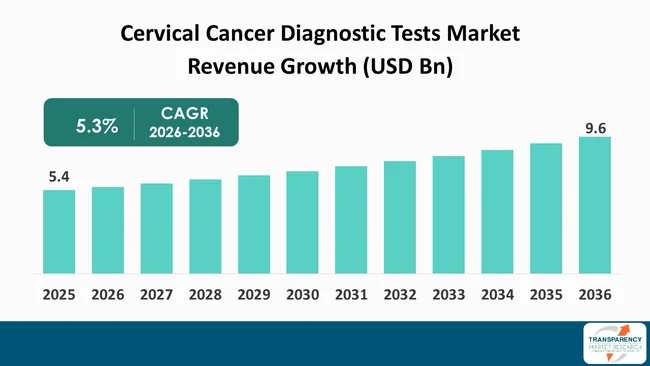
The global cervical cancer diagnostic tests market size was valued at US$ 5.4 Bn in 2025 and is projected to reach US$ 9.6 Bn by 2036, expanding at a CAGR of 5.3% from 2026 to 2036. The global market is experiencing steadiness, driven by increasing incidences of cervical cancer, rising awareness about early detection, and preventive screening programs across the globe. Key market drivers include the growing adoption of Pap smear and HPV testing, expanding government-led screening initiatives, and technological advancements in molecular diagnostics that enable faster, more accurate, and non-invasive detection of cervical cancer.
The cervical cancer diagnostic tests market is a steadily expanding market driven by public health initiatives and technological innovation. The main factors driving the market are public awareness and participation in organized screening initiatives that have increased human papillomavirus (HPV) vaccination rates, and led to follow-up testing needs, and they also benefit from molecular diagnostics including high-risk HPV DNA assays, mRNA testing, and liquid-based cytology that delivers better results through faster testing processes.
Market expansion receives support from government screening guidelines, which improve reimbursement in different areas and financial backing for women's health initiatives. The main market obstacles stem from unequal access to screening and diagnostic services in low- and middle-income countries, shortage of laboratory facilities in some areas, and the expensive nature of advanced molecular testing.
The screening uptake for particular groups is affected by both - cultural stigma and their low level of health literacy. The market offers opportunities through point-of-care platforms and self-sampling kits, which boost participation and AI-enabled tools for cytology and risk-stratification that enhance operational efficiency while decreasing the need for expert evaluation.
The ongoing market trends show that many screening programs now prefer primary HPV testing instead of cytology while self-collection methods become increasingly common, diagnostics providers merge through acquisitions, and integrated digital platforms emerge for remote follow-up and data analysis.
Cervical cancer diagnostic tests are medical tests used to detect precancerous changes or cancerous cells in the cervix at an early stage. These tests include screening methods such as the Pap smear (Pap test), human papillomavirus (HPV) testing, liquid-based cytology, colposcopy, and biopsy, which help identify abnormal cervical cells, and enable timely diagnosis, monitoring, and treatment of cervical cancer.
| Attribute | Detail |
|---|---|
| Market Drivers |
|
The market for cervical cancer diagnostic tests is bound to experience substantial growth as increased occurrence of cervical cancer and its associated precancerous conditions would create a need for early diagnosis and continuous screening procedures. The most common cancer among women throughout the world remains cervical cancer, which particularly affects women in low- and middle-income countries that lack both - preventive medical services and basic cancer awareness. The increasing occurrence of high-risk human papillomavirus (HPV) infections that serve as the main risk factor for cervical cancer development has led to a heightened disease burden.
Cervical cancer-related death rates have now decreased through regular screening as healthcare systems and public health institutions recognize early detection as a crucial method for disease prevention. Medical professionals use early diagnosis to detect precancerous cervical cell alterations that they can treat before patients reach advanced disease stages.
The medical field has seen an increased use of diagnostic tools that include Pap smears, HPV DNA tests, liquid-based cytology, colposcopy, and biopsy. Government-sponsored screening initiatives and educational outreach programs are motivating high-risk women to participate in regular testing. The rising disease rates combined with active screening programs have created conditions that drive the growth of the global cervical cancer diagnostic tests market while sustaining the need for innovative testing methods.
The increase in government-operated cervical cancer screening programs and public health initiatives is expected to create the major market growth for cervical cancer diagnostic tests. Governments and public health organizations worldwide are prioritizing women's health by establishing national screening programs that enable early cervical cancer detection and prevention methods. Many governments now include cervical cancer screening into their comprehensive preventive healthcare systems that include maternal and reproductive health programs to enhance patient accessibility.
Financial support through subsidized testing, free screening camps, and favorable reimbursement policies enables more people to access diagnostic services. The ongoing growth of public screening programs and awareness campaigns leads to increased usage of cervical cancer diagnostic tests, which results in market growth and supports the sustained development of the international market.
| Attribute | Detail |
|---|---|
| Market Opportunity |
|
The increasing adoption of home testing and self-testing solutions creates significant opportunity for the growth of cervical cancer diagnostic tests market as these methods enable users to overcome the main difficulties that prevent people from undergoing traditional hospital testing procedures.
Self-sampling kits enable women to gather cervical and vaginal samples from their homes that increases their ability to participate in screening programs as it provides them with private and more comfortable testing options. The service provides essential assistance to women who reside in remote areas due to lack of access to medical facilities and healthcare professionals who can provide proper treatment.
Home testing solutions help women who want to avoid screening tests as they suffer from social and cultural challenges, which include fear, stigma, and pelvic exam discomfort. Self-sampling methods provide a testing solution that has lower invasive requirements and greater testing convenience, which results in more people getting screened and discovering their precancerous lesions.
Molecular diagnostics technology advancements have achieved self-collected samples that can deliver reliable HPV DNA testing results matching the accuracy of samples obtained by clinicians. National screening programs now receive more support from both - governments and healthcare organizations, which enables them to roll out self-testing programs that expand screening access while decreasing the pressure on healthcare systems. Digital health systems together with telemedicine technology provide remote patient assessment and treatment services that result in improved patient participation and ongoing healthcare management. Home-based healthcare solutions gain wider acceptance from people that drives the growth of self-sampling and home-based testing solutions enabling more people to access screening services for cervical cancer. The global cervical cancer diagnostic tests market is expected to grow continuously due to this testing method.
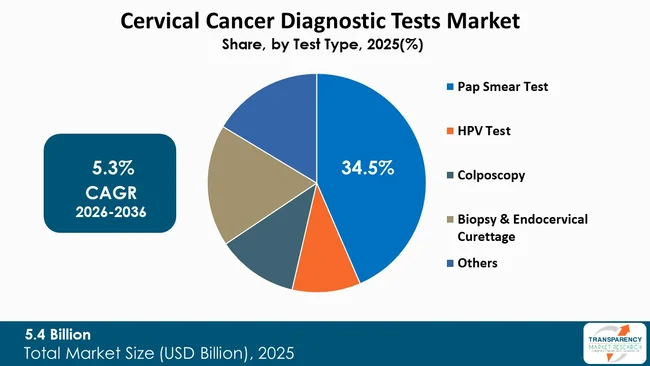
The Pap Smear Test segment, by test type, dominates the cervical cancer diagnostic tests market due to its long-standing role as the primary and most widely adopted screening method for cervical cancer. The Pap test, which is commonly known as the Pap smear, enables users to comprehensively detect early-stage precancerous and cancerous cervical cell changes through this affordable and medically validated procedure. The test has turned out to be the primary screening method used by national and regional cervical cancer prevention programs as both - healthcare professionals and patients widely have accepted it.
The Pap smear segment maintains its leading position as both - governments and established screening guidelines recommend it to healthcare providers in developed and developing nations. Many public health systems include routine Pap testing as part of women’s preventive healthcare, especially for women aged 21 and above, which ensures consistent demand.
The test achieves widespread availability as hospitals, diagnostic laboratories, and clinics provide it to users in all areas, including those with limited resources. People in low- and middle-income countries find Pap smears to be affordable diagnostic tests that medical facilities use for patient identification and treatment purposes.
The clinical reliability of Pap testing has been enhanced through the use of liquid-based cytology that produces better test results and sample materials. The Pap smear test maintains its dominant position in the global cervical cancer diagnostic tests market as it remains affordable and accessible while healthcare providers trust its clinical value.
| Attribute | Detail |
|---|---|
| Leading Region |
|
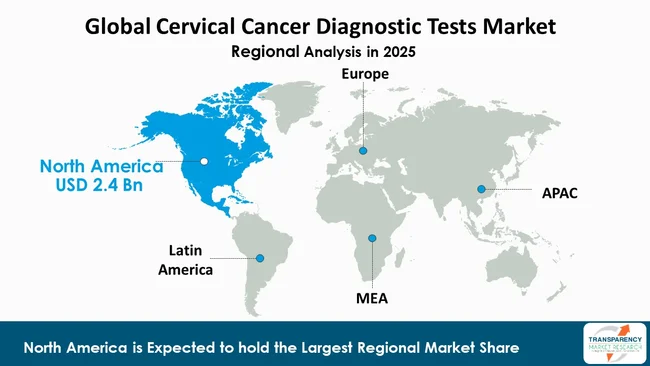
North America is the dominant region in the cervical cancer diagnostic tests market, accounting for a considerable 43.4% market share. North America leads the market due to its established healthcare systems and high awareness level regarding women's health and screening needs.
The United States and Canada provide extensive access to cutting-edge diagnostic centers and expert medical staff and advanced laboratory equipment that enables early and precise cervical cancer identification. The government implements strong national screening programs that drive higher Pap smear test and HPV test and liquid-based cytology usage through their national screening guidelines.
North America maintains its market leadership as patients can access cervical cancer screening and diagnostic tests due to favorable reimbursement policies and insurance coverage. The policies reduce patient costs while driving higher rates of participation in scheduled screening tests. The region hosts prominent diagnostic companies and research institutions that continuously invest their resources into developing next-generation molecular diagnostic methods.
Abbott, BD, COOPERSURGICAL, INC., DYSIS Medical, Inc., F. Hoffmann-La Roche Ltd., Femasys, Inc., Guided Therapeutics, Inc., Hologic, Inc., QIAGEN N.V., Quest Diagnostics Incorporated, Seegene, Inc., Arbor Vita Corporation, GenPath Diagnostics, BIOMÉRIEUX are the key players governing the global cervical cancer diagnostic tests market.
Each of these players has been profiled in the cervical cancer diagnostic tests industry research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2025 | US$ 5.4 Bn |
| Forecast Value in 2036 | More than US$ 9.6 Bn |
| CAGR | 5.3% |
| Forecast Period | 2026-2036 |
| Historical Data Available for | 2020-2024 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation | Test Type
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
The global cervical cancer diagnostic tests market was valued at US$ 5.4 Bn in 2025
The global cervical cancer diagnostic tests market is projected to cross US$ 9.6 Bn by the end of 2036
Rising prevalence of cervical cancer and precancerous lesions, increasing the need for early diagnosis and regular screening and Expansion of government-led cervical cancer screening initiatives and public health campaigns globally
It is anticipated to grow at a CAGR 5.3% from 2026 to 2036
North America is expected to account for the largest share from 2026 to 2036
Abbott, BD, COOPERSURGICAL, INC., DYSIS Medical, Inc., F. Hoffmann-La Roche Ltd., Femasys, Inc., Guided Therapeutics, Inc., Hologic, Inc., QIAGEN N.V., Quest Diagnostics Incorporated, Seegene, Inc., Arbor Vita Corporation, GenPath Diagnostics, BIOMÉRIEUX, and other prominent players
Table 01: Global Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 02: Global Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 03: Global Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 04: Global Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Country/Sub-region, 2021 to 2036
Table 05: North America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 06: North America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 07: North America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 08: North America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Country/Sub-region, 2021 to 2036
Table 09: U.S. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 10: U.S. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 11: U.S. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 12: Canada Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 13: Canada Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 14: Canada Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 15: Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 16: Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 17: Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 18: Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Country/Sub-region, 2021 to 2036
Table 19: Germany Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 20: Germany Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 21: Germany Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 22: U.K. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 23: U.K. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 24: U.K. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 25: France Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 26: France Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 27: France Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 28: Italy Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 29: Italy Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 30: Italy Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 31: Spain Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 32: Spain Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 33: Spain Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 34: The Netherlands Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 35: The Netherlands Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 36: The Netherlands Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 37: Rest of Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 38: Rest of Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 39: Rest of Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 40: Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 41: Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 42: Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 43: Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Country/Sub-region, 2021 to 2036
Table 44: China Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 45: China Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 46: China Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 47: Japan Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 48: Japan Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 49: Japan Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 50: India Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 51: India Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 52: India Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 53: South Korea Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 54: South Korea Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 55: South Korea Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 56: Australia Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 57: Australia Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 58: Australia Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 59: ASEAN Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 60: ASEAN Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 61: ASEAN Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 62: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 63: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 64: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 65: Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 66: Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 67: Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 68: Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Country/Sub-region, 2021 to 2036
Table 69: Brazil Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 70: Brazil Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 71: Brazil Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 72: Mexico Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 73: Mexico Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 74: Mexico Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 75: Argentina Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 76: Argentina Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 77: Argentina Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 78: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 79: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 80: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 81: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 82: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 83: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 84: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Country/Sub-region, 2021 to 2036
Table 85: GCC Countries Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 86: GCC Countries Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 87: GCC Countries Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 88: South Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 89: South Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 90: South Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Table 91: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Test Type, 2021 to 2036
Table 92: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by Age Group, 2021 to 2036
Table 93: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, by End-user, 2021 to 2036
Figure 01: Global Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 02: Global Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 03: Global Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 04: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Pap Smear Test, 2021 to 2036
Figure 05: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by HPV Test, 2021 to 2036
Figure 06: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Colposcopy, 2021 to 2036
Figure 07: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Biopsy & Endocervical Curettage, 2021 to 2036
Figure 08: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Others, 2021 to 2036
Figure 09: Global Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 10: Global Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 11: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Up to 35 years, 2021 to 2036
Figure 12: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by 35 - 50 years, 2021 to 2036
Figure 13: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Above 50 years, 2021 to 2036
Figure 14: Global Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 15: Global Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 16: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Hospitals, 2021 to 2036
Figure 17: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Specialty Clinics/Centers, 2021 to 2036
Figure 18: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Diagnostics Laboratories, 2021 to 2036
Figure 19: Global Cervical Cancer Diagnostic Tests Market Revenue (US$ Bn), by Others, 2021 to 2036
Figure 20: Global Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 21: Global Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 22: North America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 23: North America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 24: North America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 25: North America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 26: North America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 27: North America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 28: North America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 29: North America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 30: North America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 31: U.S. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 32: U.S. Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 33: U.S. Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 34: U.S. Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 35: U.S. Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 36: U.S. Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 37: U.S. Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 38: Canada Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 39: Canada Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 40: Canada Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 41: Canada Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 42: Canada Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 43: Canada Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 44: Canada Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 45: Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 46: Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 47: Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 48: Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 49: Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 50: Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 51: Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 52: Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 53: Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 54: Germany Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 55: Germany Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 56: Germany Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 57: Germany Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 58: Germany Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 59: Germany Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 60: Germany Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 61: U.K. Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 62: U.K. Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 63: U.K. Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 64: U.K. Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 65: U.K. Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 66: U.K. Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 67: U.K. Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 68: France Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 69: France Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 70: France Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 71: France Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 72: France Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 73: France Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 74: France Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 75: Italy Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 76: Italy Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 77: Italy Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 78: Italy Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 79: Italy Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 80: Italy Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 81: Italy Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 82: Spain Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 83: Spain Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 84: Spain Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 85: Spain Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 86: Spain Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 87: Spain Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 88: Spain Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 89: The Netherlands Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 90: The Netherlands Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 91: The Netherlands Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 92: The Netherlands Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 93: The Netherlands Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 94: The Netherlands Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 95: The Netherlands Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 96: Rest of Europe Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 97: Rest of Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 98: Rest of Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 99: Rest of Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 100: Rest of Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 101: Rest of Europe Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 102: Rest of Europe Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 103: Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 104: Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 105: Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 106: Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 107: Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 108: Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 109: Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 110: Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 111: Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 112: China Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 113: China Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 114: China Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 115: China Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 116: China Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 117: China Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 118: China Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 119: Japan Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 120: Japan Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 121: Japan Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 122: Japan Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 123: Japan Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 124: Japan Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 125: Japan Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 126: India Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 127: India Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 128: India Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 129: India Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 130: India Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 131: India Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 132: India Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 133: South Korea Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 134: South Korea Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 135: South Korea Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 136: South Korea Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 137: South Korea Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 138: South Korea Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 139: South Korea Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 140: Australia Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 141: Australia Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 142: Australia Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 143: Australia Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 144: Australia Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 145: Australia Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 146: Australia Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 147: ASEAN Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 148: ASEAN Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 149: ASEAN Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 150: ASEAN Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 151: ASEAN Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 152: ASEAN Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 153: ASEAN Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 154: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 155: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 156: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 157: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 158: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 159: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 160: Rest of Asia Pacific Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 161: Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 162: Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 163: Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 164: Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 165: Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 166: Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 167: Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 168: Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 169: Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 170: Brazil Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 171: Brazil Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 172: Brazil Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 173: Brazil Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 174: Brazil Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 175: Brazil Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 176: Brazil Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 177: Mexico Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 178: Mexico Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 179: Mexico Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 180: Mexico Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 181: Mexico Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 182: Mexico Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 183: Mexico Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 184: Argentina Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 185: Argentina Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 186: Argentina Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 187: Argentina Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 188: Argentina Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 189: Argentina Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 190: Argentina Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 191: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 192: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 193: Rest of Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 194: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 195: Rest of Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 196: Rest of Latin America Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 197: Rest of Latin America Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 198: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 199: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 200: Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 201: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 202: Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 203: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 204: Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 205: Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 206: Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 207: GCC Countries Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 208: GCC Countries Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 209: GCC Countries Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 210: GCC Countries Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 211: GCC Countries Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 212: GCC Countries Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 213: GCC Countries Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 214: South Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 215: South Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 216: South Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 217: South Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 218: South Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 219: South Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 220: South Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 221: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value (US$ Bn) Forecast, 2021 to 2036
Figure 222: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Test Type, 2025 and 2036
Figure 223: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Test Type, 2026 to 2036
Figure 224: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by Age Group, 2025 and 2036
Figure 225: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by Age Group, 2026 to 2036
Figure 226: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Value Share Analysis, by End-user, 2025 and 2036
Figure 227: Rest of Middle East and Africa Cervical Cancer Diagnostic Tests Market Attractiveness Analysis, by End-user, 2026 to 2036