Reports
Reports
Transdermal Scopolamine Market: Snapshot
Transdermal Scopolamine market is showing remarkable growth in healthcare owing to advantages offered by transdermal scopolamine. The skin patch helps prevent nausea and vomiting caused due to motion sickness by balancing norepinephrine and acetylcholine (hormones) in the body.
Application of transdermal scopolamine post-surgical procedure is propelling growth of transdermal scopolamine. It is prescribed by doctors to mitigate the effect of anesthesia, or while recovering from any surgery. Simple usability and effectiveness of the patch is making its popular among patients.
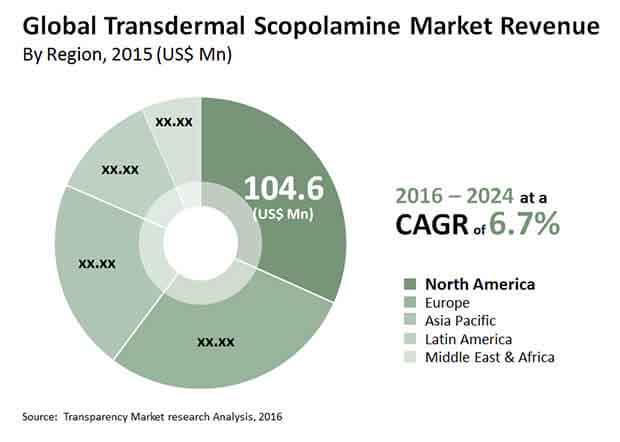
Increase in the incidence of cancer and surgeries is major driver of the transdermal scopolamine market. As transdermal scopolamine helps in recovery from anesthesia-induced as well as post-operative vomiting and nausea, the demand for transdermal scopolamine is increasing substantially around the globe. This demand accounts for market expansion at a CAGR of 6.70% between 2016 and 2024. As per the estimation by Transparency Market Research (TMR), the market value is projected to reach US$585.3 mn by the end of 2024.
The published report provides insights about the market growth. It offers detailed study of drivers and trends that will be contributing to the expansion of transdermal Scopolamine market in the duration of forecast period. The market intelligence report also gives description about the restraining factors that are likely to produce hindrance in the market. Besides, it also highlight segments which will be contributing dominantly in growth of the market globally.
The medicine can be easily self-administered at home which makes it desirable among increasing geriatric population. Increasing in geriatric population across the globe is likely to drive transdermal scopolamine market globally. Also, there is no drug administration cost involved.
Increasing Awareness about Usage of Transdermal Scopolamine among Traveler bolstering the Market
There is growth in the number of travelers around the globe. And, awareness about the benefits of transdermal scopolamine market among the travelers is bolstering the expansion of the global transdermal scopolamine market.
One of the major benefit offered by transdermal scopolamine is it can be removed when the drug is not required in the body, this factor is boosting the demand of transdermal scopolamine, thereby, pushing the horizon of transdermal scopolamine market. On the other hand, removal of transdermal scopolamine market leaves withdrawal symptoms which are found to be adverse. This is likely to produce hindrance in the growth of transdermal scopolamine market.
Owing to the increase in number of travelers in North America, the region is contribute majorly in the global transdermal scopolamine market. Also, the region has well-established healthcare infrastructure which adds in the expansion of the transdermal scopolamine market. Registering the number of surgeries occurring in Europe, the region is likely to hold second position as a contributor in the global transdermal scopolamine market.
Some of the prominent players operating in the transdermal scopolamine market are GlaxoSmithKline Plc, Caleb Pharmaceuticals Inc, Baxter International Inc., Myungmoon Pharma Co. Ltd., Novartis AG, and Perrigo Co. Plc.
Among all the key players, Novartis AG alone contributed 41.0% of the global revenue in the year 2016. At the same time, Baxter International Inc. and GlaxoSmithKline Plc., accounted for 49% of the total share cumulatively. Presence of small companies are intensifying the competition in the market.
Transdermal Scopolamine Market to Witness Notable Growth Owing to Rising Use Post-Surgery
Utilization of transdermal scopolamine post-surgery is impelling development of transdermal scopolamine. It is recommended by specialists to relieve the impact of sedation, or while recuperating from any medical procedure. Straightforward ease of use and viability of the fix is making its mainstream among patients. Utilization of transdermal scopolamine post-surgery is moving development of transdermal scopolamine. It is endorsed by specialists to moderate the impact of sedation, or while recuperating from any medical procedure. Straightforward convenience and viability of the fix is making its famous among patients.
Expanding mindfulness about the adequacy of transdermal scopolamine in the therapy of movement ailment is boosting reception of transdermal scopolamine. This factor is probably going to help the development of the worldwide transdermal scopolamine market. Furthermore, developing number of movements and explorers utilizing the transdermal scopolamine as security objects is supporting development of the worldwide transdermal scopolamine market. Inferable from the expansion in number of voyagers in North America, the district is to contribute significantly in the worldwide transdermal scopolamine market. Additionally, the area has grounded medical care framework which includes the development of the transdermal scopolamine market. Enlisting the quantity of medical procedures happening in Europe, the locale is probably going to stand firm on second footing as a donor in the worldwide transdermal scopolamine market.
Regardless, the conceivable results brought about by the utilization of the items like tiredness, hindered movement, and weakened understudies are hampering development of the worldwide transdermal scopolamine market. Furthermore, presence of tough guidelines identified with assembling and selling these items are creasing development of the worldwide transdermal scopolamine market.
All things considered, rising mindfulness in the agricultural nations are boosting request and expected to offer worthwhile development openings. The higher creation from the little and mid-scale organizations working in creating areas is making development of the worldwide transdermal scopolamine market. Likewise, improving medical care area all around the world and blasting clinical the travel industry are offering the massive potential to these organizations for development.
Chapter 1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
Chapter 2. Assumptions and Research Methodology
Chapter 3. Executive Summary: Global Transdermal Scopolamine Market
Chapter 4. Market Overview
4.1. Introduction
4.1.1. Scopolamine Indications
4.1.2. Industry Evolution / Developments
4.2. Market Overview
4.3. Key Market Indicators
4.4. Market Dynamics
4.4.1. Drivers
4.4.2. Restraints
4.4.3. Opportunities
4.5. Global Transdermal Scopolamine Market Analysis and Forecast, 2016 – 2024
4.5.1. Market Revenue Projections (US$ Mn)
4.6. Porter’s five forces analysis
4.7. Value Chain Analysis
4.8. Market Outlook
4.9. Global Company Share Analysis – 2016 (Estimated)
4.10. Scopolamine Product Prescription Rate by Region (%)
4.10.1. Oral
4.10.2. Injections
4.10.3. Transdermal / Patch
4.11. Pipeline Analysis – Transdermal Scopolamine
Chapter 5. Global Transdermal Scopolamine Market Analysis and Forecasts, By Region
5.1. Key Findings
5.2. Policies and Regulations
5.3. Market Size (US$ Mn) Forecast By Region
5.3.1. North America
5.3.2. Europe
5.3.3. Asia Pacific
5.3.4. Latin America
5.3.5. Middle East and Africa
5.4. Market Attractiveness By Region
Chapter 6. North America Transdermal Scopolamine Market Analysis and Forecast
6.1. Introduction
6.1.1. Key Findings
6.1.2. Policies and Regulations
6.1.3. Price Trend Analysis
6.1.4. Key Trends
6.2. Market Size (US$ Mn) Forecast, By Country
6.2.1. U.S.
6.2.2. Canada
6.3. Market Attractiveness Analysis By Country
Chapter 7. Europe Transdermal Scopolamine Market Analysis and Forecast
7.1. Introduction
7.1.1. Key Findings
7.1.2. Policies and Regulations
7.1.3. Price Trend Analysis
7.1.4. Key Trends
7.2. Market Size (US$ Mn) Forecast, By Country
7.2.1. Germany
7.2.2. France
7.2.3. U.K.
7.2.4. Spain
7.2.5. Italy
7.2.6. Rest of Europe
7.3. Market Attractiveness Analysis By Country
Chapter 8. Asia Pacific Transdermal Scopolamine Market Analysis and Forecast
8.1. Introduction
8.1.1. Key Findings
8.1.2. Policies and Regulations
8.1.3. Price Trend Analysis
8.1.4. Key Trends
8.2. Market Size (US$ Mn) Forecast, By Country
8.2.1. Japan
8.2.2. China
8.2.3. India
8.2.4. Australia
8.2.5. Rest of Asia Pacific
8.3. Market Attractiveness Analysis By Country
Chapter 9. Latin America Transdermal Scopolamine Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.1.2. Policies and Regulations
9.1.3. Price Trend Analysis
9.1.4. Key Trends
9.2. Market Size (US$ Mn) Forecast, By Country
9.2.1. Brazil
9.2.2. Mexico
9.2.3. Rest of Latin America
9.3. Market Attractiveness Analysis By Country
Chapter 10. Middle East & Africa Transdermal Scopolamine Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.1.2. Policies and Regulations
10.1.3. Price Trend Analysis
10.1.4. Key Trends
10.2. Market Size (US$ Mn) Forecast, By Country
10.2.1. GCC Countries
10.2.2. South Africa
10.2.3. Rest of Middle East and Africa
10.3. Market Attractiveness Analysis By Country
Chapter 11. Competition Landscape
11.1. Transdermal Scopolamine Market – Competition Matrix (By Tier and Size of companies) (2015)
11.2. Company Profiles (Details – Overview, Financials, Recent Developments, Strategy)
11.2.1. Baxter International Inc.
11.2.1.1. Company Overview (HQ, Business Segments, Employee Strength)
11.2.1.2. Product Portfolio
11.2.1.3. SWOT Analysis
11.2.1.4. Financial Overview
11.2.1.5. Strategic Overview
11.2.2. GlaxoSmithKline plc
11.2.2.1. Company Overview (HQ, Business Segments, Employee Strength)
11.2.2.2. Product Portfolio
11.2.2.3. SWOT Analysis
11.2.2.4. Financial Overview
11.2.2.5. Strategic Overview
11.2.3. Novartis AG
11.2.3.1. Company Overview (HQ, Business Segments, Employee Strength)
11.2.3.2. Product Portfolio
11.2.3.3. SWOT Analysis
11.2.3.4. Financial Overview
11.2.3.5. Strategic Overview
11.2.4. Perrigo Company plc.
11.2.4.1. Company Overview (HQ, Business Segments, Employee Strength)
11.2.4.2. Product Portfolio
11.2.4.3. SWOT Analysis
11.2.4.4. Financial Overview
11.2.4.5. Strategic Overview
11.2.5. Caleb Pharmaceuticals, Inc.
11.2.5.1. Company Overview (HQ, Business Segments, Employee Strength)
11.2.5.2. Product Portfolio
11.2.5.3. SWOT Analysis
11.2.5.4. Financial Overview
11.2.5.5. Strategic Overview
11.2.6. Myungmoon Pharma Co. LTD.
11.2.6.1. Company Overview (HQ, Business Segments, Employee Strength)
11.2.6.2. Product Portfolio
11.2.6.3. SWOT Analysis
11.2.6.4. Financial Overview
11.2.6.5. Strategic Overview
Chapter 12. Key Take Away
List of Tables
Table 01: Global Transdermal Scopolamine Market Size (US$ Mn) Forecast, 2015–2024
Table 02: Global Transdermal Scopolamine Market Size (US$ Mn) Forecast, by Region, 2015–2024
Table 03: North America Transdermal Scopolamine Market Size (US$ Mn) Forecast, by Country,
Table 04: Europe Transdermal Scopolamine Market Size (US$ Mn) Forecast, by Country / Sub-region, 2015–2024
Table 05: Asia Pacific Transdermal Scopolamine Market Size (US$ Mn) Forecast, by Country / Sub-region, 2015–2024
Table 06: Latin America Transdermal Scopolamine Market Size (US$ Mn) Forecast, by Country / Sub-region, 2015–2024
Table 07: Middle East & Africa Transdermal Scopolamine Market Size (US$ Mn) Forecast, by Country / Sub-region, 2015–2024
List of Figures
Figure 01: Global Transdermal Scopolamine Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2014–2024
Figure 02: Global Transdermal Scopolamine Market Share Analysis, by Company, 2016 (Estimated)
Figure 03: Global Scopolamine Product Prescription Share, by Region
Figure 04: Global Transdermal Scopolamine Market Value Share Analysis, by Region, 2015 and 2024
Figure 05: Global Transdermal Scopolamine Market Attractiveness Analysis, by Region
Figure 06: North America Transdermal Scopolamine Market Value Share Analysis, by Country, 2015 and 2024
Figure 07: North America Transdermal Scopolamine Market Attractiveness Analysis, by Country
Figure 08: Europe Transdermal Scopolamine Market Value Share Analysis, by Country / Sub-region, 2015 and 2024
Figure 09: Europe Transdermal Scopolamine Market Attractiveness Analysis, by Country / Sub-region
Figure 10: Asia Pacific Transdermal Scopolamine Market Value Share Analysis, by Country / Sub-region, 2015 and 2024
Figure 11: Asia Pacific Transdermal Scopolamine Market Attractiveness Analysis, by Country / Sub-region
Figure 12: Latin America Transdermal Scopolamine Market Value Share Analysis, by Country / Sub-region, 2015 and 2024
Figure 13: Latin America Transdermal Scopolamine Market Attractiveness Analysis, by Country / Sub-region
Figure 14: MEA Transdermal Scopolamine Market Value Share Analysis, by Country / Sub-region, 2015 and 2024
Figure 15: Middle East & Africa Transdermal Scopolamine Market Attractiveness Analysis, by Country / Sub-region