Reports
Reports
The global renal denervation devices market size was valued at US$ 654.5 Mn in 2025 and is projected to reach US$ 4,274.2 Mn by 2036, expanding at a CAGR of 18.6% from 2026 to 2036. The market growth is driven by rising prevalence of resistant and uncontrolled hypertension, increasing preference for minimally invasive procedures, favorable regulatory developments and approvals, and growing body of positive clinical evidence.
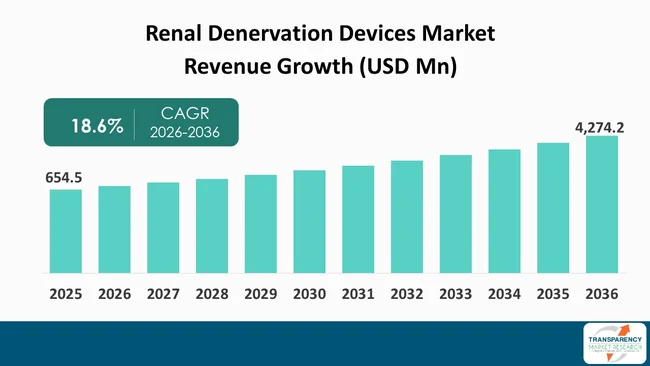
The renal denervation devices market is driven by increased incidences of hypertension, especially uncontrolled and resistant hypertension, which cannot be controlled using medication alone. There is renewed confidence among physicians about the efficacy and safety of the procedure, driven by the growing clinical evidence base from recent randomized controlled trials.
Technological advancements in catheter design and ultrasound and radiofrequency-based systems have also increased the precision of the procedure. The need for minimally-invasive treatment options and increased investments by key players in the market are also boosting the market.
The renal denervation devices market is undergoing several key trends. Firstly, a move toward more evidence-based clinical adoption has gained momentum after the release of many positive results from large randomized trials that have demonstrated long term reductions in blood pressure. Secondly, technology platforms such as radio frequency and ultrasound are being utilized along with new and emerging energy modalities that provide safer and more effective procedures.
Furthermore, manufacturers are increasingly focused on creating catheters that are simple to use and can be inserted with minimal trauma to the patient via minimally invasive techniques, as well as improving workflow with real-time imaging systems. Manufacturers are rapidly expanding into Asia and the other emerging regions due to the rise in the number of cases of hypertension globally and improvements in healthcare services in those areas. Moreover, increased partnerships among manufacturers, researchers, and the other healthcare organizations provide additional opportunities for use in clinical settings and reimbursement.
The minimally-invasive medical devices called renal denervating devices are intended to selectively disrupt the excessively active sympathetic nerve system, located along renal arteries and play an important role in blood pressure control. The renal denervation devices use energy-based methods delivered by catheter-based endovascular technologies to reduce renal sympathetic nerve activity, allowing for prolonged reductions in blood pressure. A primary clinical use of these devices is controlling hypertension, especially in patients who have either not benefited from or cannot tolerate pharmacological therapy.
| Attribute | Detail |
|---|---|
| Renal Denervation Devices Market Drivers |
|
The growing incidence of resistant and uncontrolled hypertension is a key growth driver to the renal denervation device market due to the significant unmet medical need for alternative therapeutic options.
Resistant hypertension is on the rise among the global population, especially among obese people and the elderly population. Despite the availability of pharmacological agents for the treatment of hypertension, there is a lack of response to antihypertensive drugs and noncompliance with drug regimens due to adverse effects and drug complexity.
Minimal-access renal-denervation is a catheter-based procedure that directly impacts hyper-sympathetic nerve presentation at the entrance to a renal artery and leads to significant and ongoing decreases in systolic blood pressure without requiring patient compliance with pharmacological treatment.
Many recent randomized controlled trials (RCTs) including SPYRAL HTN-OFF MED and RADIANCE-HTN support the efficacy of this approach for patients with resistant hypertension. Both of these RCTs indicate that physicians’ confidence regarding the efficacy of renal-denervation procedures has increased; therefore the growing adoption of renal-denervation by physicians will be enhanced by having supportive clinical data.
In addition, the increasing awareness of the risks associated with untreated or uncontrolled hypertension among patients, as well as healthcare practitioners, also fuels the demand for new, device-based treatment approaches. The increasing patient population with hypertension, limitations of pharmacological treatment, and the demonstrated efficacy of RDN devices combine to provide a compelling case for this treatment option, especially in regions with an exploding patient population with resistant hypertension. The increasing patient population with resistant hypertension and uncontrolled hypertension thus acts as a foundation for the RDN devices market.
The growing demand for minimally invasive procedures is another major growth driver to the renal denervation devices market. This is due to the growing preference for procedures that are less risky and require minimal recovery time. Surgical procedures for the treatment of hypertension-related complications are invasive and come with a higher risk of complications. They also require a longer recovery period and hospitalization.
On the other hand, the renal denervation procedure is a minimally invasive procedure that involves treating overactive sympathetic nerves surrounding the renal arteries for lowering blood pressure. This is done through endovascular access and involves minimal risk of complications like bleeding and infections. The minimally invasive nature of the RDN procedure reduces the risk of complications during the procedure.
The way patients are cared for has changed dramatically over the past several years due to the shift to patient-centric care with faster recovery times. As a part of this transition, doctors are now more open to studying and practicing with new treatments such as renal denervation since they can obtain results comparable to conventional medication while using less invasive methods to avoid all of the problems involved in completing the major surgeries.
Advancements in catheter design, imaging, and real-time feedback during procedures are contributing to renal denervation being more appealing for interventional radiologists, cardiologists, and nephrologists. Furthermore, physicians’ acceptance, patient demand, and healthcare system efficiency have all played a part in raising acceptance of renal denervation devices.
| Attribute | Detail |
|---|---|
| Renal Denervation Devices Market Opportunities |
|
Renal denervation’s expanded therapeutic potential is a major market growth opportunity as research continues to uncover its potential beyond the treatment of resistant hypertension.
There are also significant benefits to examine combative therapies&-combining RDN with an optimized pharmacological regimen-to help provide better treatment response in patients who only partially respond to medications. This combined method matches the shift toward personalized medicine and target-based treatment options that have been gaining prominence in modern health care. There is now an increasing number of global clinical trial studies for the expanded applications of RDN, and more providers desire to see RDN added as part of their multidisciplinary treatment plan.
There are also significant benefits to be realized by exploring combative therapies by integrating RDN with the best pharmacological intervention to assist in providing a better treatment outcome in patients who do not respond adequately to pharmacological therapy. This type of therapy aligns with the trend toward more personalized medicine and target-based therapy options that have been growing in popularity within modern medicine. There are now an increasing number of worldwide clinical trial studies for the expanded use of RDN therapy, and more practitioners want to see RDN integrated as a component of their treatment plan.
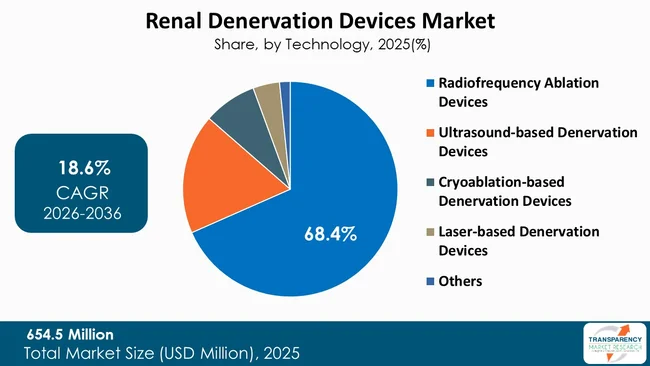
At present, the largest market segment for renal denervation devices is occupied by radiofrequency ablation devices due to its proven clinical efficacy and safety, and physicians’ comfort with the technology. Radiofrequency ablation devices have also been used to perform large-scale randomized clinical trials, such as the SPYRAL HTN OFF MED study, which demonstrate the ability to lower blood pressure in patients with resistant hypertension. Radiofrequency ablation catheters target the nerves precisely and avoid complications due to their catheter-based approach.
Furthermore, RF devices are extensively used by large medical device manufacturers in conjunction with the extensive clinical educational resources and the regulatory authorizations in the major markets, which build physicians' confidence in using RF devices. RF devices remain the most popular technique amongst healthcare providers worldwide when compared to the other newer technologies, as they provide similar efficacy, simplicity of the procedure, and evidenced efficacy over the period of time. Therefore, RF devices will continue to dominate the market regardless of the other competing technologies.
| Attribute | Detail |
|---|---|
| Leading Region |
|
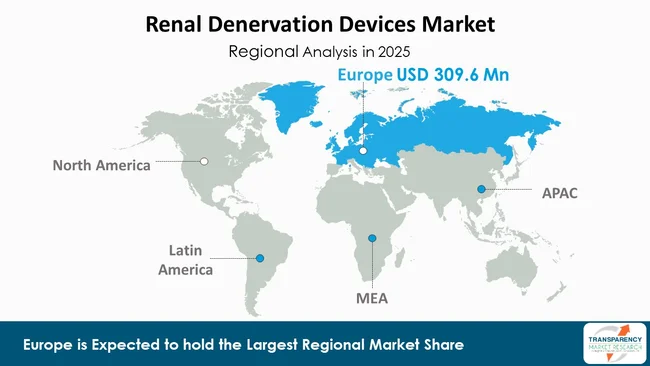
According to a new market outlook report for renal denervation devices, Europe is expected to hold 47.3% of the total market share in 2025.
Currently, the market share of the RDN devices market is dominated by the European region due to the presence of an already established healthcare system and the early adoption of innovative cardiovascular treatments. The region also has a high incidence rate of hypertension and resistant hypertension. This has led to an increased need for alternative treatments for hypertension. The region has the advantage of possessing advanced interventional cardiology and nephrology facilities. This has made the region suitable for the adoption of minimally invasive treatments.
The early and widespread approvals for the key RDN devices have also contributed to the dominance of the region. This is due to the CE marking of the RDN devices. The region also has a high level of clinical awareness and confidence among physicians due to the robust clinical trials conducted. This has been complemented by the presence of leading manufacturers of RDN devices. This has made the region the most lucrative market for the RDN devices.
The major initiatives in the renal denervation devices market include strategic partnerships, mergers and acquisitions, and collaborations with research organizations to improve clinical trials. The companies are investing in the development of new catheter technologies, geographical expansions, regulatory approvals, and training programs for physicians to improve the market position of the companies.
Medtronic, Boston Scientific Corporation, Recor Medical, Inc., Ablative Solutions, Inc., Terumo Corporation, Mercator MedSystems, Inc., Autonomix Medical, Inc., Koninklijke Philips N.V., SyMap Medical (Suzhou) Ltd., and Otsuka Medical Devices Co., Ltd. are some of the leading players operating in the global renal denervation devices market.
Each of these players has been profiled in the renal denervation devices market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2025 | US$ 654.5 Mn |
| Forecast Value in 2036 | US$ 4,274.2 Mn |
| CAGR | 18.6% |
| Forecast Period | 2026-2036 |
| Historical Data Available for | 2021-2024 |
| Quantitative Units | US$ Mn |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation | Technology
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
The global renal denervation devices market was valued at US$ 654.5 Mn in 2025
The global renal denervation devices industry is projected to reach more than US$ 4,274.2 Mn by the end of 2036
Rising prevalence of resistant and uncontrolled hypertension, increasing preference for minimally invasive procedures, favorable regulatory developments and approvals, and growing body of positive clinical evidence are some of the factors driving the expansion of renal denervation devices market.
The CAGR is anticipated to be 18.6% from 2026 to 2036
Medtronic, Boston Scientific Corporation, Recor Medical, Inc., Ablative Solutions, Inc., Terumo Corporation, Mercator MedSystems, Inc., Autonomix Medical, Inc., Koninklijke Philips N.V., SyMap Medical (Suzhou) Ltd., and Otsuka Medical Devices Co., Ltd.
Table 01: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 02: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 03: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 04: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 05: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 06: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, By Region, 2021 to 2036
Table 07: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, by Country, 2021-2036
Table 08: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 09: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 10: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 11: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 12: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 13: U.S. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 14: U.S. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 15: U.S. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 16: U.S. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 17: U.S. Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 18: Canada Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 19: Canada Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 20: Canada Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 21: Canada Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 22: Canada Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 23: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2021-2036
Table 24: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 25: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 26: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 27: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 28: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 29: Germany Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 30: Germany Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 31: Germany Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 32: Germany Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 33: Germany Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 34: U.K. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 35: U.K. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 36: U.K. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 37: U.K. Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 38: U.K. Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 39: France Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 40: France Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 41: France Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 42: France Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 43: France Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 44: Italy Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 45: Italy Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 46: Italy Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 47: Italy Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 48: Italy Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 49: Spain Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 50: Spain Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 51: Spain Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 52: Spain Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 53: Spain Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 54: The Netherlands Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 55: The Netherlands Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 56: The Netherlands Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 57: The Netherlands Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 58: The Netherlands Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 59: Rest of Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 60: Rest of Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 61: Rest of Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 62: Rest of Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 63: Rest of Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 64: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2021-2036
Table 65: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 66: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 67: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 68: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 69: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 70: China Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 71: China Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 72: China Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 73: China Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 74: China Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 75: India Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 76: India Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 77: India Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 78: India Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 79: India Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 80: Japan Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 81: Japan Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 82: Japan Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 83: Japan Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 84: Japan Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 85: South Korea Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 86: South Korea Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 87: South Korea Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 88: South Korea Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 89: South Korea Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 90: Australia & New Zealand Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 91: Australia & New Zealand Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 92: Australia & New Zealand Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 93: Australia & New Zealand Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 94: Australia & New Zealand Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 95: ASEAN Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 96: ASEAN Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 97: ASEAN Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 98: ASEAN Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 99: ASEAN Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 100: Rest of Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 101: Rest of Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 102: Rest of Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 103: Rest of Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 104: Rest of Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 105: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2021-2036
Table 106: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 107: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 108: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 109: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 110: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 111: Brazil Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 112: Brazil Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 113: Brazil Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 114: Brazil Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 115: Brazil Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 116: Argentina Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 117: Argentina Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 118: Argentina Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 119: Argentina Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 120: Argentina Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 121: Mexico Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 122: Mexico Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 123: Mexico Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 124: Mexico Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 125: Mexico Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 126: Rest of Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 127: Rest of Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 128: Rest of Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 129: Rest of Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 130: Rest of Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 131: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, by Country/Sub-region, 2021-2036
Table 132: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 133: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 134: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 135: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 136: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 137: GCC Countries Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 138: GCC Countries Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 139: GCC Countries Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 140: GCC Countries Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 141: GCC Countries Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 142: South Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 143: South Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 144: South Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 145: South Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 146: South Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Table 147: Rest of Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Technology, 2021 to 2036
Table 148: Rest of Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Product Type, 2021 to 2036
Table 149: Rest of Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Procedure Type, 2021 to 2036
Table 150: Rest of Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By Indication, 2021 to 2036
Table 151: Rest of Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, By End-user, 2021 to 2036
Figure 01: Global Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 02: Global Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 03: Global Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 04: Global Renal Denervation Devices Market Revenue (US$ Mn), by Radiofrequency Ablation Devices, 2021 to 2036
Figure 05: Global Renal Denervation Devices Market Revenue (US$ Mn), by Ultrasound-based Denervation Devices, 2021 to 2036
Figure 06: Global Renal Denervation Devices Market Revenue (US$ Mn), by Cryoablation-based Denervation Devices, 2021 to 2036
Figure 07: Global Renal Denervation Devices Market Revenue (US$ Mn), by Laser-based Denervation Devices, 2021 to 2036
Figure 08: Global Renal Denervation Devices Market Revenue (US$ Mn), by Others, 2021 to 2036
Figure 09: Global Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 10: Global Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 11: Global Renal Denervation Devices Market Revenue (US$ Mn), by Denervation Catheter Systems, 2021 to 2036
Figure 12: Global Renal Denervation Devices Market Revenue (US$ Mn), by Accessory & Consumables, 2021 to 2036
Figure 13: Global Renal Denervation Devices Market Revenue (US$ Mn), by Navigation / Imaging Support Tools, 2021 to 2036
Figure 14: Global Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 15: Global Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 16: Global Renal Denervation Devices Market Revenue (US$ Mn), by Unilateral Renal Denervation, 2021 to 2036
Figure 17: Global Renal Denervation Devices Market Revenue (US$ Mn), by Bilateral Renal Denervation, 2021 to 2036
Figure 18: Global Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 19: Global Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 20: Global Renal Denervation Devices Market Revenue (US$ Mn), by Resistant Hypertension, 2021 to 2036
Figure 21: Global Renal Denervation Devices Market Revenue (US$ Mn), by Chronic Kidney Disease (CKD)-Associated Hypertension, 2021 to 2036
Figure 22: Global Renal Denervation Devices Market Revenue (US$ Mn), by Heart Failure, 2021 to 2036
Figure 23: Global Renal Denervation Devices Market Revenue (US$ Mn), by Others, 2021 to 2036
Figure 24: Global Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 25: Global Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 26: Global Renal Denervation Devices Market Revenue (US$ Mn), by Hospitals, 2021 to 2036
Figure 27: Global Renal Denervation Devices Market Revenue (US$ Mn), by Ambulatory Surgical Centers, 2021 to 2036
Figure 28: Global Renal Denervation Devices Market Revenue (US$ Mn), by Cardiac & Specialty Clinics, 2021 to 2036
Figure 29: Global Renal Denervation Devices Market Revenue (US$ Mn), by Others, 2021 to 2036
Figure 30: Global Renal Denervation Devices Market Value Share Analysis, by Region, 2025 and 2036
Figure 31: Global Renal Denervation Devices Market Attractiveness Analysis, by Region, 2026 to 2036
Figure 32: North America Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 33: North America Renal Denervation Devices Market Value Share Analysis, by Country, 2025 and 2036
Figure 34: North America Renal Denervation Devices Market Attractiveness Analysis, by Country, 2026 to 2036
Figure 35: North America Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 36: North America Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 37: North America Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 38: North America Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 39: North America Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 40: North America Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 41: North America Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 42: North America Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 43: North America Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 44: North America Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 45: U.S. Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 46: U.S. Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 47: U.S. Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 48: U.S. Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 49: U.S. Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 50: U.S. Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 51: U.S. Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 52: U.S. Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 53: U.S. Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 54: U.S. Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 55: U.S. Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 56: Canada Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 57: Canada Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 58: Canada Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 59: Canada Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 60: Canada Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 61: Canada Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 62: Canada Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 63: Canada Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 64: Canada Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 65: Canada Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 66: Canada Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 67: Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 68: Europe Renal Denervation Devices Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 69: Europe Renal Denervation Devices Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 70: Europe Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 71: Europe Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 72: Europe Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 73: Europe Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 74: Europe Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 75: Europe Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 76: Europe Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 77: Europe Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 78: Europe Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 79: Europe Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 80: Germany Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 81: Germany Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 82: Germany Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 83: Germany Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 84: Germany Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 85: Germany Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 86: Germany Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 87: Germany Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 88: Germany Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 89: Germany Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 90: Germany Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 91: U.K. Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 92: U.K. Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 93: U.K. Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 94: U.K. Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 95: U.K. Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 96: U.K. Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 97: U.K. Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 98: U.K. Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 99: U.K. Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 100: U.K. Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 101: U.K. Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 102: France Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 103: France Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 104: France Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 105: France Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 106: France Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 107: France Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 108: France Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 109: France Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 110: France Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 111: France Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 112: France Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 113: Italy Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 114: Italy Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 115: Italy Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 116: Italy Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 117: Italy Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 118: Italy Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 119: Italy Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 120: Italy Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 121: Italy Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 122: Italy Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 123: Italy Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 124: Spain Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 125: Spain Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 126: Spain Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 127: Spain Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 128: Spain Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 129: Spain Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 130: Spain Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 131: Spain Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 132: Spain Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 133: Spain Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 134: Spain Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 135: The Netherlands Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 136: The Netherlands Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 137: The Netherlands Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 138: The Netherlands Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 139: The Netherlands Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 140: The Netherlands Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 141: The Netherlands Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 142: The Netherlands Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 143: The Netherlands Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 144: The Netherlands Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 145: The Netherlands Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 146: Rest of Europe Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 147: Rest of Europe Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 148: Rest of Europe Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 149: Rest of Europe Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 150: Rest of Europe Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 151: Rest of Europe Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 152: Rest of Europe Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 153: Rest of Europe Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 154: Rest of Europe Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 155: Rest of Europe Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 156: Rest of Europe Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 157: Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 158: Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 159: Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 160: Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 161: Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 162: Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 163: Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 164: Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 165: Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 166: Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 167: Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 168: Asia Pacific Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 169: Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 170: China Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 171: China Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 172: China Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 173: China Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 174: China Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 175: China Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 176: China Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 177: China Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 178: China Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 179: China Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 180: China Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 181: India Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 182: India Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 183: India Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 184: India Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 185: India Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 186: India Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 187: India Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 188: India Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 189: India Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 190: India Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 191: India Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 192: Japan Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 193: Japan Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 194: Japan Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 195: Japan Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 196: Japan Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 197: Japan Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 198: Japan Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 199: Japan Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 200: Japan Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 201: Japan Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 202: Japan Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 203: South Korea Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 204: South Korea Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 205: South Korea Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 206: South Korea Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 207: South Korea Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 208: South Korea Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 209: South Korea Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 210: South Korea Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 211: South Korea Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 212: South Korea Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 213: South Korea Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 214: Australia & New Zealand Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 215: Australia & New Zealand Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 216: Australia & New Zealand Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 217: Australia & New Zealand Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 218: Australia & New Zealand Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 219: Australia & New Zealand Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 220: Australia & New Zealand Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 221: Australia & New Zealand Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 222: Australia & New Zealand Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 223: Australia & New Zealand Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 224: Australia & New Zealand Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 225: ASEAN Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 226: ASEAN Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 227: ASEAN Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 228: ASEAN Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 229: ASEAN Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 230: ASEAN Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 231: ASEAN Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 232: ASEAN Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 233: ASEAN Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 234: ASEAN Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 235: ASEAN Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 236: Rest of Asia Pacific Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 237: Rest of Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 238: Rest of Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 239: Rest of Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 240: Rest of Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 241: Rest of Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 242: Rest of Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 243: Rest of Asia Pacific Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 244: Rest of Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 245: Rest of Asia Pacific Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 246: Rest of Asia Pacific Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 247: Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 248: Latin America Renal Denervation Devices Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 249: Latin America Renal Denervation Devices Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 250: Latin America Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 251: Latin America Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 252: Latin America Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 253: Latin America Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 254: Latin America Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 255: Latin America Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 256: Latin America Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 257: Latin America Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 258: Latin America Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 259: Latin America Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 260: Brazil Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 261: Brazil Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 262: Brazil Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 263: Brazil Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 264: Brazil Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 265: Brazil Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 266: Brazil Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 267: Brazil Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 268: Brazil Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 269: Brazil Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 270: Brazil Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 271: Argentina Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 272: Argentina Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 273: Argentina Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 274: Argentina Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 275: Argentina Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 276: Argentina Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 277: Argentina Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 278: Argentina Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 279: Argentina Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 280: Argentina Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 281: Argentina Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 282: Mexico Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 283: Mexico Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 284: Mexico Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 285: Mexico Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 286: Mexico Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 287: Mexico Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 288: Mexico Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 289: Mexico Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 290: Mexico Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 291: Mexico Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 292: Mexico Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 293: Rest of Latin America Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 294: Rest of Latin America Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 295: Rest of Latin America Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 296: Rest of Latin America Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 297: Rest of Latin America Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 298: Rest of Latin America Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 299: Rest of Latin America Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 300: Rest of Latin America Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 301: Rest of Latin America Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 302: Rest of Latin America Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 303: Rest of Latin America Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 304: Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 305: Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Country/Sub-region, 2025 and 2036
Figure 306: Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Country/Sub-region, 2026 to 2036
Figure 307: Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 308: Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 309: Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 310: Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 311: Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 312: Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 313: Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 314: Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 315: Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 316: Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 317: GCC Countries Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 318: GCC Countries Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 319: GCC Countries Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 320: GCC Countries Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 321: GCC Countries Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 322: GCC Countries Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 323: GCC Countries Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 324: GCC Countries Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 325: GCC Countries Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 326: GCC Countries Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 327: GCC Countries Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 328: South Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 329: South Africa Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 330: South Africa Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 331: South Africa Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 332: South Africa Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 333: South Africa Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 334: South Africa Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 335: South Africa Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 336: South Africa Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 337: South Africa Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 338: South Africa Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036
Figure 339: Rest of Middle East & Africa Renal Denervation Devices Market Value (US$ Mn) Forecast, 2021 to 2036
Figure 340: Rest of Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Technology, 2025 and 2036
Figure 341: Rest of Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Technology, 2026 to 2036
Figure 342: Rest of Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Product Type, 2025 and 2036
Figure 343: Rest of Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Product Type, 2026 to 2036
Figure 344: Rest of Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Procedure Type, 2025 and 2036
Figure 345: Rest of Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Procedure Type, 2026 to 2036
Figure 346: Rest of Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by Indication, 2025 and 2036
Figure 347: Rest of Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by Indication, 2026 to 2036
Figure 348: Rest of Middle East & Africa Renal Denervation Devices Market Value Share Analysis, by End-user, 2025 and 2036
Figure 349: Rest of Middle East & Africa Renal Denervation Devices Market Attractiveness Analysis, by End-user, 2026 to 2036