Reports
Reports
Chronic Obstructive Pulmonary Disease Treatment Market - Snapshot
The global chronic obstructive pulmonary disease (COPD) treatment market is driven by increase in the number of COPD cases, rise in FDA approvals, and launch of several products in the U.S. Moreover, increase in the geriatric population and rise in expenditure on pharmaceuticals in emerging countries are the other factors contributing to the growth of the market. However, patent expiry of branded products and availability of generic equivalents and alternative treatment options are anticipated to restrain the global market.
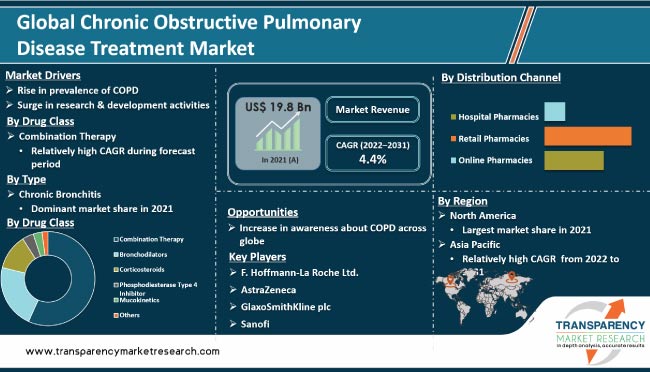
Based on drug class, the global chronic obstructive pulmonary disease treatment market has been segmented into combination, bronchodilators, corticosteroids, phosphodiesterase type 4 inhibitors, mucokinetics, and others. The combination segment has been classified into long acting muscarinic antagonist & inhaled corticosteroids (LAMA-ICS), long acting beta agonist & inhaled corticosteroids (LABA-ICS), triple therapy, and others. The bronchodilators segment has been divided into long acting beta agonist (LABA), short acting beta agonist (SABA), and long acting muscarinic antagonist (LAMA). The combination segment is projected to account for major share of the market by 2026 due to increase in number of physicians prescribing combination therapy and surge in the number of drugs available for COPD treatment. The bronchodilators segment is anticipated to grow at a steady pace owing to increase in the adoption of long acting muscarinic antagonist (LAMA) drugs. In terms of distribution channel, the market has been categorized into hospital pharmacies, retail pharmacies, and online pharmacies. The retail pharmacies segment is expected to expand at the fastest CAGR due to surge in the number of patients preferring retail pharmacies. The online pharmacies segment is likely to be driven by rise in awareness about these pharmacies among the general population.
Based on region, the global chronic obstructive pulmonary disease (COPD) treatment market has been segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America accounted for the largest share of the global market in 2017. Presence of established pharmaceutical companies, increase in adoption of new drugs launched in the market, and focus on launch of new products through R&D are anticipated to drive the market in the region during the forecast period.
The chronic obstructive pulmonary disease (COPD) treatment market in Europe is driven by increase in R&D expenditure and launch of new products. In November 2018, GlaxoSmithKline plc and Innoviva, Inc. received authorization from the European Commission for expanded use for once-daily Trelegy Ellipta, first single inhaler triple therapy indicated for COPD patients who were not adequately treated with dual bronchodilation.
Rise in prevalence of COPD, high environmental pollution, increase in the geriatric population, and growth of the pharmaceutical industry are anticipated to fuel the growth of the chronic obstructive pulmonary disease (COPD) treatment market in Asia Pacific. An article published in the International Journal of Pulmonary and Respiratory Sciences stated that COPD is the third leading cause of death in India.
The chronic obstructive pulmonary disease (COPD) treatment market in Latin America and Middle East & Africa is expected to be driven by increase in awareness about the diagnosis and treatment of COPD, rise in the number of government initiatives to spread awareness and importance about its treatment, and surge in the number of specialty care centers for respiratory care in various countries in the Middle East. Additionally, GCC Countries such as the UAE and Saudi Arabia present significant opportunities in the market due to strengthening health care system and availability of quality care in hospitals.
Major players operating in the global chronic obstructive pulmonary disease (COPD) treatment market include AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., GlaxoSmithKline plc, Novartis AG, CHIESI Farmaceutici S.p.A., Sunovion Pharmaceuticals, Inc. (Sumitomo Dainippon Pharma Co., Ltd), Teva Pharmaceutical Industries Ltd., Mylan N.V., and Orion Corporation.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global COPD Treatment Market
4. Market Overview
4.1. Introduction
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global COPD Treatment Market Analysis and Forecast, 2016–2026
5. Key Insights
5.1. Innovative Solutions for COPD Treatment
5.2. Pipeline Analysis
6. Global COPD Treatment Market Analysis and Forecast, by Drug Class
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Drug Class, 2016–2026
6.3.1. Combination
6.3.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
6.3.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
6.3.1.3. Triple Therapy
6.3.1.4. Others
6.3.2. Corticosteroids
6.3.3. Bronchodilators
6.3.3.1. Long Acting Beta Agonist (LABA)
6.3.3.2. Short Acting Beta Agonist (SABA)
6.3.3.3. Long Acting Muscarinic Antagonist (LAMA)
6.3.4. Phosphodiesterase Type 4 Inhibitors
6.3.5. Mucokinetics
6.3.6. Others
6.4. Market Attractiveness, by Drug Class
7. Global COPD Treatment Market Analysis and Forecast, by Distribution Channel
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Distribution Channel, 2016–2026
7.3.1. Hospital Pharmacies
7.3.2. Retail Pharmacies
7.3.3. Online Pharmacies
7.4. Market Attractiveness, by Distribution Channel
8. Global COPD Treatment Market Analysis and Forecast, by Region
8.1. Key Findings
8.2. Market Value Forecast, by Region
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Latin America
8.2.5. Middle East & Africa
8.3. Market Attractiveness By Region
9. North America COPD Treatment Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value Forecast, by Drug Class, 2016–2026
9.2.1. Combination
9.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
9.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
9.2.1.3. Triple Therapy
9.2.1.4. Others
9.2.2. Corticosteroids
9.2.3. Bronchodilators
9.2.3.1. Long Acting Beta Agonist (LABA)
9.2.3.2. Short Acting Beta Agonist (SABA)
9.2.3.3. Long Acting Muscarinic Antagonist (LAMA)
9.2.4. Phosphodiesterase Type 4 Inhibitors
9.2.5. Mucokinetics
9.2.6. Others
9.3. Market Value Forecast, by Distribution Channel, 2016–2026
9.3.1. Hospital Pharmacies
9.3.2. Retail Pharmacies
9.3.3. Online Pharmacies
9.4. Market Value Forecast, by Country, 2016–2026
9.4.1. U.S.
9.4.2. Canada
9.5. Market Attractiveness Analysis
9.5.1. By Drug Class
9.5.2. By Distribution Channel
9.5.3. By Country
10. Europe COPD Treatment Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Drug Class, 2016–2026
10.2.1. Combination
10.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
10.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
10.2.1.3. Triple Therapy
10.2.1.4. Others
10.2.2. Corticosteroids
10.2.3. Bronchodilators
10.2.3.1. Long Acting Beta Agonist (LABA)
10.2.3.2. Short Acting Beta Agonist (SABA)
10.2.3.3. Long Acting Muscarinic Antagonist (LAMA)
10.2.4. Phosphodiesterase Type 4 Inhibitors
10.2.5. Mucokinetics
10.2.6. Others
10.3. Market Value Forecast, by Distribution Channel, 2016–2026
10.3.1. Hospital Pharmacies
10.3.2. Retail Pharmacies
10.3.3. Online Pharmacies
10.4. Market Value Forecast, by Country/Sub-region, 2016–2026
10.4.1. Germany
10.4.2. U.K.
10.4.3. France
10.4.4. Spain
10.4.5. Italy
10.4.6. Rest of Europe
10.5. Market Attractiveness Analysis
10.5.1. By Drug Class
10.5.2. By Distribution Channel
10.5.3. By Country/Sub-region
11. Asia Pacific COPD Treatment Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Drug Class, 2016–2026
11.2.1. Combination
11.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
11.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
11.2.1.3. Triple Therapy
11.2.1.4. Others
11.2.2. Corticosteroids
11.2.3. Bronchodilators
11.2.3.1. Long Acting Beta Agonist (LABA)
11.2.3.2. Short Acting Beta Agonist (SABA)
11.2.3.3. Long Acting Muscarinic Antagonist (LAMA)
11.2.4. Phosphodiesterase Type 4 Inhibitors
11.2.5. Mucokinetics
11.2.6. Others
11.3. Market Value Forecast, by Distribution Channel, 2016–2026
11.3.1. Hospital Pharmacies
11.3.2. Retail Pharmacies
11.3.3. Online Pharmacies
11.4. Market Value Forecast, by Country/Sub-region, 2016–2026
11.4.1. China
11.4.2. Japan
11.4.3. India
11.4.4. Australia & New Zealand
11.4.5. Rest of Asia Pacific
11.5. Market Attractiveness Analysis
11.5.1. By Drug Class
11.5.2. By Distribution Channel
11.5.3. By Country/Sub-region
12. Latin America COPD Treatment Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Drug Class, 2016–2026
12.2.1. Combination
12.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
12.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
12.2.1.3. Triple Therapy
12.2.1.4. Others
12.2.2. Corticosteroids
12.2.3. Bronchodilators
12.2.3.1. Long Acting Beta Agonist (LABA)
12.2.3.2. Short Acting Beta Agonist (SABA)
12.2.3.3. Long Acting Muscarinic Antagonist (LAMA)
12.2.4. Phosphodiesterase Type 4 Inhibitors
12.2.5. Mucokinetics
12.2.6. Others
12.3. Market Value Forecast, by Distribution Channel, 2016–2026
12.3.1. Hospital Pharmacies
12.3.2. Retail Pharmacies
12.3.3. Online Pharmacies
12.4. Market Value Forecast, by Country/Sub-region, 2016–2026
12.4.1. Brazil
12.4.2. Mexico
12.4.3. Rest of Latin America
12.5. Market Attractiveness Analysis
12.5.1. By Drug Class
12.5.2. By Distribution Channel
12.5.3. By Country/Sub-region
13. Middle East & Africa COPD Treatment Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Drug Class, 2016–2026
13.2.1. Combination
13.2.1.1. Long Acting Muscarinic Antagonist & Inhaled Corticosteroids (LAMA-ICS)
13.2.1.2. Long Acting Beta Agonist & Inhaled Corticosteroids (LABA-ICS)
13.2.1.3. Triple Therapy
13.2.1.4. Others
13.2.2. Corticosteroids
13.2.3. Bronchodilators
13.2.3.1. Long Acting Beta Agonist (LABA)
13.2.3.2. Short Acting Beta Agonist (SABA)
13.2.3.3. Long Acting Muscarinic Antagonist (LAMA)
13.2.4. Phosphodiesterase Type 4 Inhibitors
13.2.5. Mucokinetics
13.2.6. Others
13.3. Market Value Forecast, by Distribution Channel, 2016–2026
13.3.1. Hospital Pharmacies
13.3.2. Retail Pharmacies
13.3.3. Online Pharmacies
13.4. Market Value Forecast, by Country/Sub-region, 2016–2026
13.4.1. GCC Countries
13.4.2. South Africa
13.4.3. Rest of Middle East & Africa
13.5. Market Attractiveness Analysis
13.5.1. By Drug Class
13.5.2. By Distribution Channel
13.5.3. By Country/Sub-region
14. Competition Landscape
14.1. Market Player - Competition Matrix (By Tier and Size of companies)
14.2. Market Share Analysis, by Company, 2017
14.3. Company Profiles
14.3.1. AstraZeneca
14.3.2. Boehringer Ingelheim Pharmaceuticals, Inc.
14.3.3. GlaxoSmithKline plc
14.3.4. Novartis AG
14.3.5. Teva Pharmaceutical Industries Ltd.
14.3.6. Sunovion Pharmaceuticals, Inc. (Sumitomo Dainippon Pharma Co., Ltd.)
14.3.7. CHIESI Farmaceutici S.p.A.
14.3.8. Orion Corporation
14.3.9. Mylan N.V.
List of Tables
Table 01 Pipeline Analysis
Table 02 Pipeline Analysis
Table 03 Pipeline Analysis
Table 04 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Drug Class, 2016–2026
Table 05 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Combination Therapy, 2016–2026
Table 06 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Bronchodilators, 2016–2026
Table 07 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Distribution Channel, 2016–2026
Table 08 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Region, 2016–2026
Table 09 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Drug Class, 2016–2026
Table 10 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Combination Therapy, 2016–2026
Table 11 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Bronchodilators, 2016–2026
Table 12 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Distribution Channel, 2016–2026
Table 13 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Country, 2016–2026
Table 14 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Drug Class, 2016–2026
Table 15 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Combination Therapy, 2016–2026
Table 16 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Bronchodilators, 2016–2026
Table 17 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Distribution Channel, 2016–2026
Table 18 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 19 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Drug Class, 2016–2026
Table 20 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Combination Therapy, 2016–2026
Table 21 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Bronchodilators, 2016–2026
Table 22 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Distribution Channel, 2016–2026
Table 23 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 24 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Drug Class, 2016–2026
Table 25 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Combination Therapy, 2016–2026
Table 26 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Bronchodilators, 2016–2026
Table 27 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Distribution Channel, 2016–2026
Table 28 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 29 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Drug Class, 2016–2026
Table 30 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Combination Therapy, 2016–2026
Table 31 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Bronchodilators, 2016–2026
Table 32 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Distribution Channel, 2016–2026
Table 33 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
List of Figures
Figure 01 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Distribution, by Region, 2017 and 2026
Figure 02 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Revenue (US$ Mn), by Drug Class, 2017
Figure 03 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 04 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2017 and 2026
Figure 05 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Y-o-Y Growth, by Combination, 2016–2026
Figure 06 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Y-o-Y Growth, by Bronchodilators, 2016–2026
Figure 07 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Y-o-Y Growth, by Corticosteroids, 2016–2026
Figure 08 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Y-o-Y Growth, by Phosphodiesterase Type 4 Inhibitors, 2016–2026
Figure 09 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Y-o-Y Growth, by Mucokinetics, 2016–2026
Figure 10 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) and Y-o-Y Growth, by Others, 2016–2026
Figure 11 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2018–2026
Figure 12 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2017 and 2026
Figure 13 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, by Hospital Pharmacies, 2016–2026
Figure 14 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, by Independent Pharmacies, 2016–2026
Figure 15 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, by Online Pharmacies, 2016–2026
Figure 16 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2018–2026
Figure 17 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Region, 2017 and 2026
Figure 18 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Region, 2018–2026
Figure 19 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 20 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2017 and 2026
Figure 21 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2018–2026
Figure 22 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2017 and 2026
Figure 23 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2018 - 2026
Figure 24 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country, 2017 and 2026
Figure 25 North America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country, 2018–2026
Figure 26 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 27 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2017 and 2026
Figure 28 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2018–2026
Figure 29 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2017 and 2026
Figure 30 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2018 - 2026
Figure 31 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2017 and 2026
Figure 32 Europe Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2018–2026
Figure 33 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 34 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2017 and 2026
Figure 35 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2018–2026
Figure 36 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2017 and 2026
Figure 37 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2018–2026
Figure 38 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2017 and 2026
Figure 39 Asia Pacific Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2018–2026
Figure 40 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 41 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2017 and 2026
Figure 42 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2018–2026
Figure 43 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2017 and 2026
Figure 44 Latin America Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2018–2026
Figure 45 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 46 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Drug Class, 2017 and 2026
Figure 47 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Drug Class, 2018–2026
Figure 48 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Distribution Channel, 2017 and 2026
Figure 49 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Distribution Channel, 2018–2026
Figure 50 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Value Share Analysis, by Country/Sub-region, 2017 and 2026
Figure 51 Middle East & Africa Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Attractiveness Analysis, by Country/Sub-region, 2018–2026
Figure 52 Global Chronic Obstructive Pulmonary Disease (COPD) Treatment Market Share, by Company, 2017
Figure 53 AstraZeneca Respiratory Segment Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 54 AstraZeneca R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 55 AstraZeneca Breakdown of Net Sales (%), by Region, 2017
Figure 56 AstraZeneca Breakdown of Net Sales (%), by Product Segment, 2017
Figure 57 Boehringer Ingelheim International GmbH , Human Pharmaceuticals Business Segment Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 58 Boehringer Ingelheim International GmbH R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 59 Boehringer Ingelheim International GmbH Breakdown of Net Sales (%), by Region, 2017
Figure 60 Boehringer Ingelheim International GmbH Breakdown of Net Sales (%), by Business Segment, 2017
Figure 61 GlaxoSmithKline plc Respiratory Business Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 62 GlaxoSmithKline plc R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 63 GlaxoSmithKline plc Breakdown of Net Sales (%), by Region, 2017
Figure 64 GlaxoSmithKline plc Breakdown of Net Sales (%), by Business Segment, 2017
Figure 65 Novartis AG COPD Portfolio Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 66 Novartis AG R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 67 Novartis AG Breakdown of Net Sales (%), by Country, 2017
Figure 68 Novartis AG Breakdown of Net Sales (%), by Business Segment, 2017
Figure 69 Teva Pharmaceutical Industries Ltd Respiratory Medicine Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 70 Teva Pharmaceutical Industries Ltd R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 71 Teva Pharmaceutical Industries Ltd Breakdown of Net Sales (%), by Region, 2017
Figure 72 Teva Pharmaceutical Industries Ltd Breakdown of Net Sales (%), by Business Segment, 2017
Figure 73 Sumitomo Dainippon Pharma Co., Ltd Pharmaceuticals Segment Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 74 Sumitomo Dainippon Pharma Co., Ltd R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 75 Sumitomo Dainippon Pharma Co., Ltd Breakdown of Net Sales (%), by Region, 2017
Figure 76 Sumitomo Dainippon Pharma Co., Ltd Breakdown of Net Sales (%), by Product Segment, 2017
Figure 77 CHIESI Farmaceutici S.p.A. Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 78 CHIESI Farmaceutici S.p.A. R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 79 CHIESI Farmaceutici S.p.A. Breakdown of Net Sales (%), by Region, 2017
Figure 80 CHIESI Farmaceutici S.p.A. Breakdown of Net Sales (%), by Therapeutics Area, 2017
Figure 81 Orion Corporation Pharmaceuticals Segment Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 82 Orion Corporation R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2014–2017
Figure 83 Orion Corporation Breakdown of Net Sales (%), by Country, 2017
Figure 84 Orion Corporation Breakdown of Net Sales (%), by Business Segment, 2017
Figure 85 Mylan N.V. Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 86 Mylan N.V. R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 87 Mylan N.V. Breakdown of Net Sales (%), by Region, 2017