Reports
Reports
Analyst Viewpoint
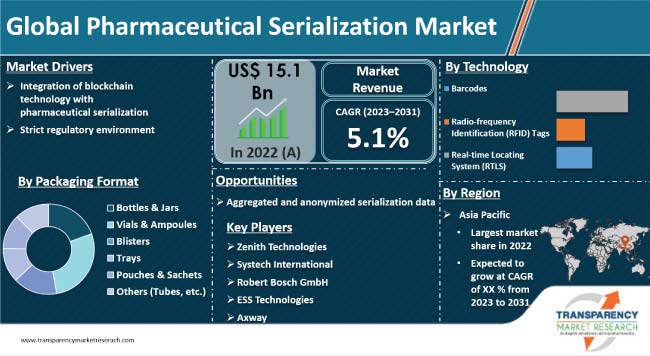
Strict regulatory environment across the world is driving the global pharmaceutical serialization market. Governments have developed efficient and effective methods against drug counterfeiting that affects public’s health. Increase in pharmaceutical counterfeiting is a major factor propelling market expansion. Furthermore, integration of blockchain technology is expected to bolster the global pharmaceutical serialization industry size during the forecast.
Development of efficient and secure drug serialization solutions offers lucrative opportunities to pharmaceutical serialization market players. Pharmaceutical companies are focusing on formulating effective serialization strategy in order to protect their products and ensure compliance with emerging regulatory requirements.
Pharmaceutical serialization is the tracing and tracking of prescription drugs with the help of the supply chain, from manufacturing to dispensing.
Changes in healthcare regulations, policies, and standards, both at international and national level, could directly impact pharmaceutical serialization. In order to enhance serialization efforts, new compliance requirements or strict regulations need to be set in the pharmaceutical industry.
The unique codes on the packaging should be readable within the supply chain to trace the pharmaceutical product. It is important to check quality of the 2D code printed and the quality of human-readable codes on the packaging.
For 2D codes, companies/manufacturers could employ ISO 15415 and OCV/OCR technology for human readable codes. For each type of packaging, it is necessary to use different qualities of image-processing equipment.
A peer-to-peer computerized technology is used for decentralized ledger blockchain. These transactions are arranged into a succession of blocks, each containing information on its own. If a given connection breaks, then the chain becomes unacceptable.
Blockchain system represents a kind of immutable distributed database; after data has been placed, it cannot be altered. The block normally has an embedded timestamp, a one-time Unique ID, along with other details such as a transaction list.
High degree of transparency provides for detailed information that can serve monitoring purposes. In the pharmaceutical industry, the network depends on the record-keeping capability of the nodes.
Stringent regulatory initiatives, such as the Drug Supply Chain Security Act (DSCSA) in the USA and the Falsified Medicines Directive (FMD) in Europe, are driving the global pharmaceutical serialization market demand.
These regulations require using serialization technologies in order for one to achieve traceability as well as authenticity in the distribution of genuine medication products, thus reducing the possibility of counterfeit drugs making their way into the supply chain.
Visibility of the supply chain is improved with real-time tracking and monitoring on different distribution networks that track the serialized pharmaceutical products. This transparency aids in the optimization of supply chain efficiencies leading to better inventory management, less stocks out, few stocks, and smooth logistics operation that ultimately enhance overall operability.
Track-and-trace products can be deployed from the point of packaging to the pharmacy. These technologies help to determine the past and current locations of the product. It does not provide a complete solution; however, it helps to minimize the interference attempts within the supply chain.
Barcodes and radio-frequency identification are two common technologies used to deliver traceability. These tags contain electronically stored information. There are two forms of RFID – active and passive RFID devices. Active tags may operate hundreds of meters and have a local power source from RFID reader, whereas passive tags interrogate radio waves by collecting energy from nearby RFID readers.
Track-and-trace technologies provide secure transmission of correct information between the supply chain network and the users. These also create a bridge to the IoT and devices such as RFID transmitters. This is a technology whereby digital data encoded in RFID tags or a reader via radio waves securely and digitally captures smart labels.
In term of packaging format, the bottles & jars accounted for the largest global pharmaceutical serialization market share in 2022. This is ascribed to excellent durability and tear & puncture resistance properties.
Serialization is being adopted by pharmaceutical companies to meet strict regulatory regulations and achieve traceability in a supply chain, with bottles and jars being essential drivers for this change. Each bottle or jar has a unique identifier which improves the accuracy of tracking, reduces the possibility of falsification, and leads to increased safety while using drugs.
As per pharmaceutical serialization market analysis, Asia Pacific is projected to dominate the global industry during the forecast period. This is ascribed to increase in consumption of pharmaceutical serialization in the different end-use industries.
According to DIA Global Forum, the first ASEAN member state to introduce pilot implementation of serialization is Indonesia. All prescription items must be serialized by the year 2025 with GTIN, marketing authorization number (MA number), serial number, expiration date, and lot number included in each GS1 authentication barcode.
The pharmaceutical serialization market report concludes with the company profiles section, which includes key information about major players in the global market.
Zenith Technologies, Systech International, Robert Bosch GmbH, ESS Technologies, Axway, Tracelink, Inc., Adents International, Antares Vision SRL, Mettler Toledo International, Inc., Impinj, Inc., Optel Group, ATL Security Label Systems, SEA VISION S.r.l, Alien Technology Corp., and SICPA Holdings are the prominent players in the global pharmaceutical serialization market.
| Attribute | Detail |
|---|---|
| Market Size Value in 2023 | US$ 15.1 Bn |
| Market Forecast Value in 2031 | US$ 24.8 Bn |
| Growth Rate (CAGR) | 5.1% |
| Forecast Period | 2023-2031 |
| Quantitative Units | US$ Bn for Value, Units for Volume |
| Market Analysis | It includes cross-segment analysis as well as regional level analysis. Moreover, the qualitative analysis includes drivers, restraints, opportunities, key trends, and a parent industry overview. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Market Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 15.1 Bn in 2022.
It is projected to grow at a CAGR of 5.1% during the forecast period.
It is expected to reach US$ 24.8 Bn by 2031.
Integration of blockchain technology with pharmaceutical serialization and strict regulatory environment
The bottles & jars segment held largest share in 2022.
Asia Pacific is projected to experience high demand for pharmaceutical serialization during the forecast period.
Zenith Technologies, Systech International, Robert Bosch GmbH, ESS Technologies, Axway, Tracelink, Inc., Adents International, Antares Vision SRL, Mettler Toledo International, Inc., Impinj, Inc., Optel Group, ATL Security Label Systems, SEA VISION S.r.l, Alien Technology Corp., and SICPA Holdings are the prominent players in the global market.
1. Executive Summary
1.1. Market Overview
1.2. Market Analysis
1.3. TMR Analysis and Recommendations
2. Market Viewpoint
2.1. Market Definition
2.2. Market Taxonomy
3. Pharmaceutical Serialization Market Overview
3.1. Global Packaging Market Overview
3.2. Global Temperature Controlled Packaging Market Overview
3.3. Macro-economic Factors – Correlation Analysis
3.4. Forecast Factors – Relevance & Impact
3.5. Market Value Chain Analysis
3.5.1. Exhaustive List of Active Participants
3.5.1.1. Manufacturers
3.5.1.2. Distributors/Retailers
3.5.1.3. End-users
3.5.2. Profitability Margins
3.6. Impact of COVID-19
3.6.1. Current Statistics and Probable Future Impact
3.6.2. Impact of COVID-19 on Pharmaceutical Serialization Market
4. Global Pharmaceutical Serialization Market Analysis
4.1. Pricing Analysis
4.1.1. Pricing Assumption
4.1.2. Price Projections, by Region
4.2. Market Size (US$ Bn) and Forecast
4.2.1. Market Size and Y-o-Y Growth
4.2.2. Absolute $ Opportunity
5. Global Pharmaceutical Serialization Market Dynamics
5.1. Drivers
5.2. Restraints
5.3. Opportunity Analysis
5.4. Trends
6. Global Pharmaceutical Serialization Market Analysis and Forecast, by Technology
6.1. Introduction
6.1.1. Market share and Basis Points (BPS) Analysis, by Technology
6.1.2. Y-o-Y Growth Projections, by Technology
6.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Technology
6.2.1. Barcodes
6.2.2. Radio-frequency Identification (RFID) Tags
6.2.3. Real-time Locating System (RTLS)
6.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Technology
6.3.1. Barcodes
6.3.2. Radio-frequency Identification (RFID) Tags
6.3.3. Real-time Locating System (RTLS)
6.4. Market Attractiveness Analysis, by Technology
7. Global Pharmaceutical Serialization Market Analysis and Forecast, by Packaging Format
7.1. Introduction
7.1.1. Market share and Basis Points (BPS) Analysis, by Packaging Format
7.1.2. Y-o-Y Growth Projections, by Packaging Format
7.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Packaging Format
7.2.1. Bottles & Jars
7.2.2. Vials & Ampoules
7.2.3. Blisters
7.2.4. Trays
7.2.5. Pouches & Sachets
7.2.6. Others (tubes, etc.)
7.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Packaging Format
7.3.1. Bottles & Jars
7.3.2. Vials & Ampoules
7.3.3. Blisters
7.3.4. Trays
7.3.5. Pouches & Sachets
7.3.6. Others (tubes, etc.)
7.4. Market Attractiveness Analysis, by Packaging Format
8. Global Pharmaceutical Serialization Market Analysis and Forecast, by End-use
8.1. Introduction
8.1.1. Market share and Basis Points (BPS) Analysis, by End-use
8.1.2. Y-o-Y Growth Projections, by End-use
8.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by End-use
8.2.1. Pharma & Biological
8.2.2. Medical & Supplies
8.2.2.1. Gloves
8.2.2.2. Scissors
8.2.2.3. Syringes & Needles
8.2.2.4. Surgical Tapes
8.2.2.5. Others
8.2.3. Medical Equipment
8.2.3.1. Surgical
8.2.3.2. Therapeutic
8.2.3.3. Diagnostic
8.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by End-use
8.3.1. Pharma & Biological
8.3.2. Medical & Supplies
8.3.2.1. Gloves
8.3.2.2. Scissors
8.3.2.3. Syringes & Needles
8.3.2.4. Surgical Tapes
8.3.2.5. Others
8.3.3. Medical Equipment
8.3.3.1. Surgical
8.3.3.2. Therapeutic
8.3.3.3. Diagnostic
8.4. Market Attractiveness Analysis, by End-use
9. Global Pharmaceutical Serialization Market Analysis and Forecast, by Region
9.1. Introduction
9.1.1. Market share and Basis Points (BPS) Analysis, by Region
9.1.2. Y-o-Y Growth Projections, by Region
9.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Region
9.2.1. North America
9.2.2. Latin America
9.2.3. Europe
9.2.4. Asia Pacific
9.2.5. Middle East & Africa
9.3. Market Size (US$ Bn) and Volume (Tons) Forecast Analysis 2023-2031 by Region
9.3.1. North America
9.3.2. Latin America
9.3.3. Europe
9.3.4. Asia Pacific
9.3.5. Middle East & Africa
9.4. Market Attractiveness Analysis, by Region
10. North America Pharmaceutical Serialization Market Analysis and Forecast
10.1. Introduction
10.1.1. Market share and Basis Points (BPS) Analysis, by Country
10.1.2. Y-o-Y Growth Projections, by Country
10.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Country
10.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Country
10.3.1. U.S.
10.3.2. Canada
10.4. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Technology
10.5. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Technology
10.5.1. Barcodes
10.5.2. Radio-frequency Identification (RFID) Tags
10.5.3. Real-time Locating System (RTLS)
10.6. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Packaging Format
10.7. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Packaging Format
10.7.1. Bottles & Jars
10.7.2. Vials & Ampoules
10.7.3. Blisters
10.7.4. Trays
10.7.5. Pouches & Sachets
10.7.6. Others (tubes, etc.)
10.8. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by End-use
10.9. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by End-use
10.9.1. Pharma & Biological
10.9.2. Medical & Supplies
10.9.2.1. Gloves
10.9.2.2. Scissors
10.9.2.3. Syringes & Needles
10.9.2.4. Surgical Tapes
10.9.2.5. Others
10.9.3. Medical Equipment
10.9.3.1. Surgical
10.9.3.2. Therapeutic
10.9.3.3. Diagnostic
10.10. Pharmaceutical Serialization Market Attractiveness Analysis
10.10.1. By Country
10.10.2. By Technology
10.10.3. By Packaging Format
10.10.4. By End-use
10.11. Drivers and Restraints: Impact Analysis
11. Latin America Pharmaceutical Serialization Market Analysis and Forecast
11.1. Introduction
11.1.1. Market share and Basis Points (BPS) Analysis, by Country
11.1.2. Y-o-Y Growth Projections, by Country
11.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Country
11.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031 by Country
11.3.1. Brazil
11.3.2. Mexico
11.3.3. Argentina
11.3.4. Rest of Latin America
11.4. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Technology
11.5. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Technology
11.5.1. Barcodes
11.5.2. Radio-frequency Identification (RFID) Tags
11.5.3. Real-time Locating System (RTLS)
11.6. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Packaging Format
11.7. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Packaging Format
11.7.1. Bottles & Jars
11.7.2. Vials & Ampoules
11.7.3. Blisters
11.7.4. Trays
11.7.5. Pouches & Sachets
11.7.6. Others (tubes, etc.)
11.8. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by End-use
11.9. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by End-use
11.9.1. Pharma & Biological
11.9.2. Medical & Supplies
11.9.2.1. Gloves
11.9.2.2. Scissors
11.9.2.3. Syringes & Needles
11.9.2.4. Surgical Tapes
11.9.2.5. Others
11.9.3. Medical Equipment
11.9.3.1. Surgical
11.9.3.2. Therapeutic
11.9.3.3. Diagnostic
11.10. Pharmaceutical Serialization Market Attractiveness Analysis
11.10.1. By Country
11.10.2. By Technology
11.10.3. By Packaging Format
11.10.4. By End-use
11.11. Drivers and Restraints: Impact Analysis
12. Europe Pharmaceutical Serialization Market Analysis and Forecast
12.1. Introduction
12.1.1. Market share and Basis Points (BPS) Analysis, by Country
12.1.2. Y-o-Y Growth Projections, by Country
12.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Country
12.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031 by Country
12.3.1. Germany
12.3.2. Spain
12.3.3. Italy
12.3.4. France
12.3.5. U.K.
12.3.6. BENELUX
12.3.7. Nordic
12.3.8. Russia
12.3.9. Poland
12.3.10. Rest of Europe
12.4. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Technology
12.5. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Technology
12.5.1. Barcodes
12.5.2. Radio-frequency Identification (RFID) Tags
12.5.3. Real-time Locating System (RTLS)
12.6. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Packaging Format
12.7. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Packaging Format
12.7.1. Bottles & Jars
12.7.2. Vials & Ampoules
12.7.3. Blisters
12.7.4. Trays
12.7.5. Pouches & Sachets
12.7.6. Others (tubes, etc.)
12.8. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by End-use
12.9. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by End-use
12.9.1. Pharma & Biological
12.9.2. Medical & Supplies
12.9.2.1. Gloves
12.9.2.2. Scissors
12.9.2.3. Syringes & Needles
12.9.2.4. Surgical Tapes
12.9.2.5. Others
12.9.3. Medical Equipment
12.9.3.1. Surgical
12.9.3.2. Therapeutic
12.9.3.3. Diagnostic
12.10. Pharmaceutical Serialization Market Attractiveness Analysis
12.10.1. By Country
12.10.2. By Technology
12.10.3. By Packaging Format
12.10.4. By End-use
12.11. Drivers and Restraints: Impact Analysis
13. Asia Pacific Pharmaceutical Serialization Market Analysis and Forecast
13.1. Introduction
13.1.1. Market share and Basis Points (BPS) Analysis, by Country
13.1.2. Y-o-Y Growth Projections, by Country
13.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Country
13.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031 by Country
13.3.1. China
13.3.2. India
13.3.3. Japan
13.3.4. ASEAN
13.3.5. Australia and New Zealand
13.3.6. Rest of Asia Pacific
13.4. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Technology
13.5. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Technology
13.5.1. Barcodes
13.5.2. Radio-frequency Identification (RFID) Tags
13.5.3. Real-time Locating System (RTLS)
13.6. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Packaging Format
13.7. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Packaging Format
13.7.1. Bottles & Jars
13.7.2. Vials & Ampoules
13.7.3. Blisters
13.7.4. Trays
13.7.5. Pouches & Sachets
13.7.6. Others (tubes, etc.)
13.8. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by End-use
13.9. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by End-use
13.9.1. Pharma & Biological
13.9.2. Medical & Supplies
13.9.2.1. Gloves
13.9.2.2. Scissors
13.9.2.3. Syringes & Needles
13.9.2.4. Surgical Tapes
13.9.2.5. Others
13.9.3. Medical Equipment
13.9.3.1. Surgical
13.9.3.2. Therapeutic
13.9.3.3. Diagnostic
13.10. Pharmaceutical Serialization Market Attractiveness Analysis
13.10.1. By Country
13.10.2. By Technology
13.10.3. By Packaging Format
13.10.4. By End-use
13.11. Drivers and Restraints: Impact Analysis
14. Middle East and Africa Pharmaceutical Serialization Market Analysis and Forecast
14.1. Introduction
14.1.1. Market share and Basis Points (BPS) Analysis, by Country
14.1.2. Y-o-Y Growth Projections, by Country
14.2. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Country
14.3. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031 by Country
14.3.1. GCC Countries
14.3.2. Northern Africa
14.3.3. South Africa
14.3.4. Turkey
14.3.5. Rest of Middle East & Africa
14.4. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Technology
14.5. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Technology
14.5.1. Barcodes
14.5.2. Radio-frequency Identification (RFID) Tags
14.5.3. Real-time Locating System (RTLS)
14.6. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by Packaging Format
14.7. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by Packaging Format
14.7.1. Bottles & Jars
14.7.2. Vials & Ampoules
14.7.3. Blisters
14.7.4. Trays
14.7.5. Pouches & Sachets
14.7.6. Others (tubes, etc.)
14.8. Historical Market Value (US$ Bn) and Volume (Units), 2018-2022, by End-use
14.9. Market Size (US$ Bn) and Volume (Units) Forecast Analysis 2023-2031, by End-use
14.9.1. Pharma & Biological
14.9.2. Medical & Supplies
14.9.2.1. Gloves
14.9.2.2. Scissors
14.9.2.3. Syringes & Needles
14.9.2.4. Surgical Tapes
14.9.2.5. Others
14.9.3. Medical Equipment
14.9.3.1. Surgical
14.9.3.2. Therapeutic
14.9.3.3. Diagnostic
14.10. Pharmaceutical Serialization Market Attractiveness Analysis
14.10.1. By Country
14.10.2. By Technology
14.10.3. By Packaging Format
14.10.4. By End-use
14.11. Drivers and Restraints: Impact Analysis
15. Country-wise Pharmaceutical Serialization Market Analysis and Forecast
15.1. U.S. Pharmaceutical Serialization Market Analysis 2023 & 2031
15.1.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.1.1.1. By Technology
15.1.1.2. By Packaging Format
15.1.1.3. By End-use
15.2. Canada Pharmaceutical Serialization Market Analysis 2023 & 2031
15.2.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.2.1.1. By Technology
15.2.1.2. By Packaging Format
15.2.1.3. By End-use
15.3. Germany Pharmaceutical Serialization Market Analysis 2023 & 2031
15.3.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.3.1.1. By Technology
15.3.1.2. By Packaging Format
15.3.1.3. By End-use
15.4. France Pharmaceutical Serialization Market Analysis 2023 & 2031
15.4.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.4.1.1. By Technology
15.4.1.2. By Packaging Format
15.4.1.3. By End-use
15.5. UK Pharmaceutical Serialization Market Analysis 2023 & 2031
15.5.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.5.1.1. By Technology
15.5.1.2. By Packaging Format
15.5.1.3. By End-use
15.6. Italy Pharmaceutical Serialization Market Analysis 2023 & 2031
15.6.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.6.1.1. By Technology
15.6.1.2. By Packaging Format
15.6.1.3. By End-use
15.7. Japan Pharmaceutical Serialization Market Analysis 2023 & 2031
15.7.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.7.1.1. By Technology
15.7.1.2. By Packaging Format
15.7.1.3. By End-use
15.8. India Pharmaceutical Serialization Market Analysis 2023 & 2031
15.8.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.8.1.1. By Technology
15.8.1.2. By Packaging Format
15.8.1.3. By End-use
15.9. China Pharmaceutical Serialization Market Analysis 2023 & 2031
15.9.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.9.1.1. By Technology
15.9.1.2. By Packaging Format
15.9.1.3. By End-use
15.10. Australia Pharmaceutical Serialization Market Analysis 2023 & 2031
15.10.1. Market Volume and Value (US$ Bn) Analysis and Forecast by Market Taxonomy
15.10.1.1. By Technology
15.10.1.2. By Packaging Format
15.10.1.3. By End-use
15.11. Drivers and Restraints: Impact Analysis
16. Competitive Landscape
16.1. Competition Dashboard
16.2. Company Market Share Analysis
16.3. Competition Deep Dive (Global Players)
16.3.1. Zenith Technologies
16.3.1.1. Overview
16.3.1.2. Financials
16.3.1.3. Strategy
16.3.1.4. Recent Developments
16.3.1.5. SWOT Analysis(The same will be provided for all the companies)
16.3.2. Systech International
16.3.2.1. Overview
16.3.2.2. Financials
16.3.2.3. Strategy
16.3.2.4. Recent Developments
16.3.2.5. SWOT Analysis(The same will be provided for all the companies)
16.3.3. Robert Bosch GmbH
16.3.3.1. Overview
16.3.3.2. Financials
16.3.3.3. Strategy
16.3.3.4. Recent Developments
16.3.3.5. SWOT Analysis(The same will be provided for all the companies)
16.3.4. ESS Technologies
16.3.4.1. Overview
16.3.4.2. Financials
16.3.4.3. Strategy
16.3.4.4. Recent Developments
16.3.4.5. SWOT Analysis(The same will be provided for all the companies)
16.3.5. Axway
16.3.5.1. Overview
16.3.5.2. Financials
16.3.5.3. Strategy
16.3.5.4. Recent Developments
16.3.5.5. SWOT Analysis(The same will be provided for all the companies)
16.3.6. Tracelink Inc.
16.3.6.1. Overview
16.3.6.2. Financials
16.3.6.3. Strategy
16.3.6.4. Recent Developments
16.3.6.5. SWOT Analysis(The same will be provided for all the companies)
16.3.7. Adents International
16.3.7.1. Overview
16.3.7.2. Financials
16.3.7.3. Strategy
16.3.7.4. Recent Developments
16.3.7.5. SWOT Analysis(The same will be provided for all the companies)
16.3.8. Antares Vision SRL
16.3.8.1. Overview
16.3.8.2. Financials
16.3.8.3. Strategy
16.3.8.4. Recent Developments
16.3.8.5. SWOT Analysis(The same will be provided for all the companies)
16.3.9. Mettler Toledo International Inc.
16.3.9.1. Overview
16.3.9.2. Financials
16.3.9.3. Strategy
16.3.9.4. Recent Developments
16.3.9.5. SWOT Analysis(The same will be provided for all the companies)
16.3.10. Impinj Inc.
16.3.10.1. Overview
16.3.10.2. Financials
16.3.10.3. Strategy
16.3.10.4. Recent Developments
16.3.10.5. SWOT Analysis(The same will be provided for all the companies)
16.3.11. Optel Group
16.3.11.1. Overview
16.3.11.2. Financials
16.3.11.3. Strategy
16.3.11.4. Recent Developments
16.3.11.5. SWOT Analysis(The same will be provided for all the companies)
16.3.12. ATL Security Label Sysytems
16.3.12.1. Overview
16.3.12.2. Financials
16.3.12.3. Strategy
16.3.12.4. Recent Developments
16.3.12.5. SWOT Analysis(The same will be provided for all the companies)
16.3.13. SEA VISION S.r.l
16.3.13.1. Overview
16.3.13.2. Financials
16.3.13.3. Strategy
16.3.13.4. Recent Developments
16.3.13.5. SWOT Analysis(The same will be provided for all the companies)
16.3.14. Alien Technology Corp.
16.3.14.1. Overview
16.3.14.2. Financials
16.3.14.3. Strategy
16.3.14.4. Recent Developments
16.3.14.5. SWOT Analysis(The same will be provided for all the companies)
16.3.15. SICPA Holdings
16.3.15.1. Overview
16.3.15.2. Financials
16.3.15.3. Strategy
16.3.15.4. Recent Developments
16.3.15.5. SWOT Analysis(The same will be provided for all the companies)
17. Assumptions and Acronyms Used
18. Research Methodology
List of Tables
Table 01: Global Pharmaceutical Serialization Market Historic Value (US$ Bn), by Packaging Format 2018(H)-2022(A)
Table 02: Global Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Packaging Format 2023(E)-2031(F)
Table 03: Global Pharmaceutical Serialization Market Historic Volume (Units), by Packaging Format 2018(H)-2022(A)
Table 04: Global Pharmaceutical Serialization Market Forecast Volume (Units), by Packaging Format 2023(E)-2031(F)
Table 05: Global Pharmaceutical Serialization Market Historic Value (US$ Bn), by Technology 2018(H)-2022(A)
Table 06: Global Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Technology 2023(E)-2031(F)
Table 07: Global Pharmaceutical Serialization Market Historic Volume (Units), by Technology 2018(H)-2022(A)
Table 08: Global Pharmaceutical Serialization Market Forecast Volume (Units), by Technology 2023(E)-2031(F)
Table 09: Global Pharmaceutical Serialization Market Historic Value (US$ Bn), by End use 2018(H)-2022(A)
Table 10: Global Pharmaceutical Serialization Market Forecast Value (US$ Bn), by End use 2023(E)-2031(F)
Table 11: Global Pharmaceutical Serialization Market Historic Volume (Units), by End use 2018(H)-2022(A)
Table 12: Global Pharmaceutical Serialization Market Forecast Volume (Units), by End use 2023(E)-2031(F)
Table 13: Global Pharmaceutical Serialization Market Historic Value (US$ Bn), By Region 2018(H)-2022(A)
Table 14: Global Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Region 2023(E)-2031(F)
Table 15: Global Pharmaceutical Serialization Market Historic Volume (Units), by Region 2018(H)-2022(A)
Table 16: Global Pharmaceutical Serialization Market Forecast Volume (Units), by Region 2023(E)-2031(F)
Table 17: North America Pharmaceutical Serialization Market Historic Value (US$ Bn), by Packaging Format 2018(H)-2022(A)
Table 18: North America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Packaging Format 2023(E)-2031(F)
Table 19: North America Pharmaceutical Serialization Market Historic Volume (Units), by Packaging Format 2018(H)-2022(A)
Table 20: North America Pharmaceutical Serialization Market Forecast Volume (Units), by Packaging Format 2023(E)-2031(F)
Table 21: North America Pharmaceutical Serialization Market Historic Value (US$ Bn), by Technology 2018(H)-2022(A)
Table 22: North America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Technology 2023(E)-2031(F)
Table 23: North America Pharmaceutical Serialization Market Historic Volume (Units), by Technology 2018(H)-2022(A)
Table 24: North America Pharmaceutical Serialization Market Forecast Volume (Units), by Technology 2023(E)-2031(F)
Table 25: North America Pharmaceutical Serialization Market Historic Value (US$ Bn), by End use 2018(H)-2022(A)
Table 26: North America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by End use 2023(E)-2031(F)
Table 27: North America Pharmaceutical Serialization Market Historic Volume (Units), by End use 2018(H)-2022(A)
Table 28: North America Pharmaceutical Serialization Market Forecast Volume (Units), by End use 2023(E)-2031(F)
Table 29: North America Pharmaceutical Serialization Market Historic Value (US$ Bn), by Country 2018(H)-2022(A)
Table 30: North America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Country 2023(E)-2031(F)
Table 31: North America Pharmaceutical Serialization Market Historic Volume (Units), by Country 2018(H)-2022(A)
Table 32: North America Pharmaceutical Serialization Market Forecast Volume (Units), by Country 2023(E)-2031(F)
Table 31: Latin America Pharmaceutical Serialization Market Historic Value (US$ Bn), by Packaging Format 2018(H)-2022(A)
Table 34: Latin America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Packaging Format 2023(E)-2031(F)
Table 35: Latin America Pharmaceutical Serialization Market Historic Volume (Units), by Packaging Format 2018(H)-2022(A)
Table 36: Latin America Pharmaceutical Serialization Market Forecast Volume (Units), by Packaging Format 2023(E)-2031(F)
Table 37: Latin America Pharmaceutical Serialization Market Historic Value (US$ Bn), by Technology 2018(H)-2022(A)
Table 38: Latin America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Technology 2023(E)-2031(F)
Table 39: Latin America Pharmaceutical Serialization Market Historic Volume (Units), by Technology 2018(H)-2022(A)
Table 40: Latin America Pharmaceutical Serialization Market Forecast Volume (Units), by Technology 2023(E)-2031(F)
Table 41: Latin America Pharmaceutical Serialization Market Historic Value (US$ Bn), by End use 2018(H)-2022(A)
Table 42: Latin America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by End use 2023(E)-2031(F)
Table 43: Latin America Pharmaceutical Serialization Market Historic Volume (Units), by End use 2018(H)-2022(A)
Table 44: Latin America Pharmaceutical Serialization Market Forecast Volume (Units), by End use 2023(E)-2031(F)
Table 45: Latin America Pharmaceutical Serialization Market Historic Value (US$ Bn), by Country 2018(H)-2022(A)
Table 46: Latin America Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Country 2023(E)-2031(F)
Table 47: Latin America Pharmaceutical Serialization Market Historic Volume (Units), by Country 2018(H)-2022(A)
Table 48: Latin America Pharmaceutical Serialization Market Forecast Volume (Units), by Country 2023(E)-2031(F)
Table 49: Europe Pharmaceutical Serialization Market Historic Value (US$ Bn), by Packaging Format 2018(H)-2022(A)
Table 50: Europe Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Packaging Format 2023(E)-2031(F)
Table 51: Europe Pharmaceutical Serialization Market Historic Volume (Units), by Packaging Format 2018(H)-2022(A)
Table 52: Europe Pharmaceutical Serialization Market Forecast Volume (Units), by Packaging Format 2023(E)-2031(F)
Table 53: Europe Pharmaceutical Serialization Market Historic Value (US$ Bn), by Technology 2018(H)-2022(A)
Table 54: Europe Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Technology 2023(E)-2031(F)
Table 55: Europe Pharmaceutical Serialization Market Historic Volume (Units), by Technology 2018(H)-2022(A)
Table 56: Europe Pharmaceutical Serialization Market Forecast Volume (Units), by Technology 2023(E)-2031(F)
Table 57: Europe Pharmaceutical Serialization Market Historic Value (US$ Bn), by End use 2018(H)-2022(A)
Table 58: Europe Pharmaceutical Serialization Market Forecast Value (US$ Bn), by End use 2023(E)-2031(F)
Table 59: Europe Pharmaceutical Serialization Market Historic Volume (Units), by End use 2018(H)-2022(A)
Table 60: Europe Pharmaceutical Serialization Market Forecast Volume (Units), by End use 2023(E)-2031(F)
Table 61: Europe Pharmaceutical Serialization Market Historic Value (US$ Bn), by Country 2018(H)-2022(A)
Table 62: Europe Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Country 2023(E)-2031(F)
Table 63: Europe Pharmaceutical Serialization Market Historic Volume (Units), by Country 2018(H)-2022(A)
Table 64: Europe Pharmaceutical Serialization Market Forecast Volume (Units), by Country 2023(E)-2031(F)
Table 65: Asia Pacific Pharmaceutical Serialization Market Historic Value (US$ Bn), by Packaging Format 2018(H)-2022(A)
Table 66: Asia Pacific Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Packaging Format 2023(E)-2031(F)
Table 67: Asia Pacific Pharmaceutical Serialization Market Historic Volume (Units), by Packaging Format 2018(H)-2022(A)
Table 68: Asia Pacific Pharmaceutical Serialization Market Forecast Volume (Units), by Packaging Format 2023(E)-2031(F)
Table 69: Asia Pacific Pharmaceutical Serialization Market Historic Value (US$ Bn), by Technology 2018(H)-2022(A)
Table 70: Asia Pacific Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Technology 2023(E)-2031(F)
Table 71: Asia Pacific Pharmaceutical Serialization Market Historic Volume (Units), by Technology 2018(H)-2022(A)
Table 72: Asia Pacific Pharmaceutical Serialization Market Forecast Volume (Units), by Technology 2023(E)-2031(F)
Table 73: Asia Pacific Pharmaceutical Serialization Market Historic Value (US$ Bn), by End use 2018(H)-2022(A)
Table 74: Asia Pacific Pharmaceutical Serialization Market Forecast Value (US$ Bn), by End use 2023(E)-2031(F)
Table 75: Asia Pacific Pharmaceutical Serialization Market Historic Volume (Units), by End use 2018(H)-2022(A)
Table 76: Asia Pacific Pharmaceutical Serialization Market Forecast Volume (Units), by End use 2023(E)-2031(F)
Table 77: Asia Pacific Pharmaceutical Serialization Market Historic Value (US$ Bn), by Country 2018(H)-2022(A)
Table 78: Asia Pacific Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Country 2023(E)-2031(F)
Table 79: Asia Pacific Pharmaceutical Serialization Market Historic Volume (Units), by Country 2018(H)-2022(A)
Table 80: Asia Pacific Pharmaceutical Serialization Market Forecast Volume (Units), by Country 2023(E)-2031(F)
Table 81: Middle East & Africa Pharmaceutical Serialization Market Historic Value (US$ Bn), by Packaging Format 2018(H)-2022(A)
Table 82: Middle East & Africa Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Packaging Format 2023(E)-2031(F)
Table 83: Middle East & Africa Pharmaceutical Serialization Market Historic Volume (Units), by Packaging Format 2018(H)-2022(A)
Table 84: Middle East & Africa Pharmaceutical Serialization Market Forecast Volume (Units), by Packaging Format 2023(E)-2031(F)
Table 85: Middle East & Africa Pharmaceutical Serialization Market Historic Value (US$ Bn), by Technology 2018(H)-2022(A)
Table 86: Middle East & Africa Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Technology 2023(E)-2031(F)
Table 87: Middle East & Africa Pharmaceutical Serialization Market Historic Volume (Units), by Technology 2018(H)-2022(A)
Table 88: Middle East & Africa Pharmaceutical Serialization Market Forecast Volume (Units), by Technology 2023(E)-2031(F)
Table 89: Middle East & Africa Pharmaceutical Serialization Market Historic Value (US$ Bn), by End use 2018(H)-2022(A)
Table 90: Middle East & Africa Pharmaceutical Serialization Market Forecast Value (US$ Bn), by End use 2023(E)-2031(F)
Table 91: Middle East & Africa Pharmaceutical Serialization Market Historic Volume (Units), by End use 2018(H)-2022(A)
Table 92: Middle East & Africa Pharmaceutical Serialization Market Forecast Volume (Units), by End use 2023(E)-2031(F)
Table 93: Middle East & Africa Pharmaceutical Serialization Market Historic Value (US$ Bn), by Country 2018(H)-2022(A)
Table 94: Middle East & Africa Pharmaceutical Serialization Market Forecast Value (US$ Bn), by Country 2023(E)-2031(F)
Table 95: Middle East & Africa Pharmaceutical Serialization Market Historic Volume (Units), by Country 2018(H)-2022(A)
Table 96: Middle East & Africa Pharmaceutical Serialization Market Forecast Volume (Units), by Country 2023(E)-2031(F)
List of Figures
Figure 01: Global Pharmaceutical Serialization Market Share Analysis by Packaging Format, 2023E & 2031F
Figure 02: Global Pharmaceutical Serialization Market Attractiveness Analysis by Packaging Format, 2023E-2031F
Figure 03: Global Pharmaceutical Serialization Market Y-o-Y Analysis by Packaging Format, 2018H-2031F
Figure 04: Global Pharmaceutical Serialization Market Share Analysis by Technology, 2023E & 2031F
Figure 05: Global Pharmaceutical Serialization Market Attractiveness Analysis by Technology, 2023E-2031F
Figure 06: Global Pharmaceutical Serialization Market Y-o-Y Analysis by Technology, 2018H-2031F
Figure 07: Global Pharmaceutical Serialization Market Share Analysis by End use, 2023E & 2031F
Figure 08: Global Pharmaceutical Serialization Market Attractiveness Analysis by End use, 2023E-2031F
Figure 09: Global Pharmaceutical Serialization Market Y-o-Y Analysis by End use, 2018H-2031F
Figure 10: Global Pharmaceutical Serialization Market Share Analysis by Region, 2023E & 2031F
Figure 11: Global Pharmaceutical Serialization Market Attractiveness Analysis by Region, 2023E-2031F
Figure 12: Global Pharmaceutical Serialization Market Y-o-Y Analysis by Region, 2018H-2031F
Figure 13: North America Pharmaceutical Serialization Market Value Share Analysis by Packaging Format 2023(E)
Figure 14: North America Pharmaceutical Serialization Market Value Share Analysis by Technology 2023(E)
Figure 15: North America Pharmaceutical Serialization Market Attractiveness Analysis by End use, 2023E-2031F
Figure 16: North America Pharmaceutical Serialization Market Value Share Analysis by Country 2023(E)
Figure 17: Latin America Pharmaceutical Serialization Market Value Share Analysis by Packaging Format 2023(E)
Figure 18: Latin America Pharmaceutical Serialization Market Value Share Analysis by Technology 2023(E)
Figure 19: Latin America Pharmaceutical Serialization Market Attractiveness Analysis by End use, 2023E-2031F
Figure 20: Latin America Pharmaceutical Serialization Market Value Share Analysis by Country 2023(E)
Figure 21: Europe Pharmaceutical Serialization Market Value Share Analysis by Packaging Format 2023(E)
Figure 22: Europe Pharmaceutical Serialization Market Value Share Analysis by Technology 2023(E)
Figure 23: Europe Pharmaceutical Serialization Market Attractiveness Analysis by End use, 2023E-2031F
Figure 24: Europe Pharmaceutical Serialization Market Value Share Analysis by Country 2023(E)
Figure 25: Asia Pacific Pharmaceutical Serialization Market Value Share Analysis by Packaging Format 2023(E)
Figure 26: Asia Pacific Pharmaceutical Serialization Market Value Share Analysis by Technology 2023(E)
Figure 27: Asia Pacific Pharmaceutical Serialization Market Attractiveness Analysis by End use, 2023E-2031F
Figure 28: Asia Pacific Pharmaceutical Serialization Market Value Share Analysis by Country 2023(E)
Figure 29: Middle East & Africa Pharmaceutical Serialization Market Value Share Analysis by Packaging Format 2023(E)
Figure 30: Middle East & Africa Pharmaceutical Serialization Market Value Share Analysis by Technology 2023(E)
Figure 31: Middle East & Africa Pharmaceutical Serialization Market Attractiveness Analysis by End use, 2023E-2031F
Figure 32: Middle East & Africa Pharmaceutical Serialization Market Value Share Analysis by Country 2023(E)
Figure 31: U.S. Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 34: U.S. Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 35: U.S. Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 36: Canada Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 37: Canada Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 38: Canada Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 39: Brazil Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 40: Brazil Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 41: Brazil Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 42: Mexico Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 43: Mexico Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 44: Mexico Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 45: Germany Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 46: Germany Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 47: Germany Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 48: Spain Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 49: Spain Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 50: Spain Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 51: France Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 52: France Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 53: France Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 54: U.K. Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 55: U.K. Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 56: U.K. Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 57: Italy Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 58: Italy Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 59: Italy Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 60: Russia Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 61: Russia Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 62: Russia Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 63: China Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 64: China Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 65: China Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 66: India Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 67: India Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 68: India Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 69: Japan Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 70: Japan Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 71: Japan Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 72: GCC Countries Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 73: GCC Countries Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 74: GCC Countries Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F
Figure 75: South Africa Pharmaceutical Serialization Market Value Share Analysis, by Packaging Format, 2023E
Figure 76: South Africa Pharmaceutical Serialization Market Value Share Analysis, by Technology, 2023E
Figure 77: South Africa Pharmaceutical Serialization Market Value Share Analysis, by End use, 2023E & 2031F