Reports
Reports
Analysts’ Viewpoint
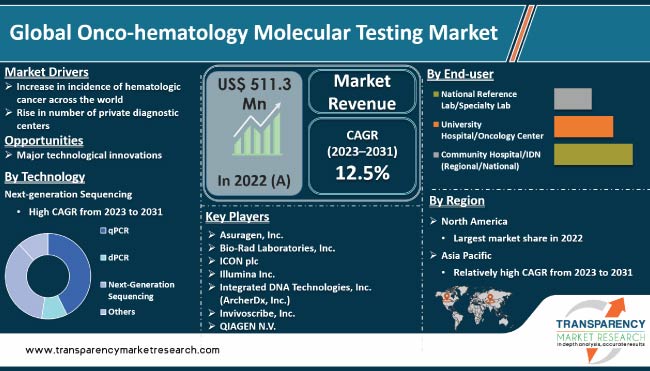
Increase in incidence of blood cancers, rise in awareness about early diagnosis, advancements in testing technologies, and development of targeted therapies are driving the global onco-hematology molecular testing market. Onco-hematology molecular testing refers to diagnostic tests used in the field of hematological oncology that focus on the diagnosis and treatment of blood cancers and related disorders. Rise in awareness among healthcare providers and patients about the importance of early and accurate diagnosis in improving treatment outcomes is expected to propel market expansion in the next few years.
Rise in prevalence of blood cancers and advancements in diagnostic technologies offer lucrative opportunities to market players. Companies are constantly investing in research & development activities to introduce innovative tests and technologies in order to enhance accuracy, speed, and cost-effectiveness of onco-hematology molecular testing.
Hemato oncology tests are diagnostic procedures used in the field of hematological oncology to detect and monitor blood cancers and related disorders. These tests play a crucial role in the early detection, diagnosis, and treatment of hematological malignancies such as leukemia, lymphoma, and myeloma.
Hemato oncology testing involves various techniques, including molecular testing, flow cytometry, cytogenetics, and next-generation sequencing. These tests help identify genetic mutations, chromosomal abnormalities, and other molecular markers associated with different types of blood cancers. By analyzing these markers, healthcare professionals can make accurate diagnoses, determine disease progression, and tailor treatment plans to individual patients.
Importance of onco-hematology molecular testing has grown significantly in the past few years, driven by advancements in testing technologies and development of targeted therapies. These tests provide valuable information for personalized medicine approaches, helping healthcare providers optimize treatment strategies and improve patient outcomes.
Overall, hemato oncology tests are vital tools in the fight against blood cancer, enabling early detection, precise diagnosis, and effective monitoring of these complex diseases. Continued advancements in testing techniques are expected to enhance the accuracy and efficiency of hemato oncology testing in the near future.
Surge in incidence of hematologic cancer across the world is likely to propel the global onco-hematology molecular testing market size during the forecast period. Hematologic cancers, which include leukemia, lymphoma, and myeloma, are a major health concern globally, with increasing numbers of cases being reported each year.
Several factors contribute to the rise in hematologic cancer incidence. These include aging population, environmental factors, exposure to carcinogens, genetic predisposition, and lifestyle changes. Additionally, advancements in medical technology and rise in awareness about early detection and diagnosis have led to improved detection rates of hematologic cancers.
Surge in incidence of hematologic cancers has led to rise in need for accurate and efficient diagnostic tools. Onco-hematology molecular testing plays a crucial role in the diagnosis, prognosis, and monitoring of these diseases. These tests enable healthcare professionals to identify specific genetic mutations, chromosomal abnormalities, and other molecular markers associated with different types of hematologic cancers.
Rise in demand for early and accurate diagnosis is projected to bolster global onco-hematology molecular testing market growth in the near future. Companies are investing in research & development activities to introduce innovative testing technologies, improve the speed & accuracy of tests, and enhance the overall patient experience.
In conclusion, increase in incidence of hematologic cancer across the world is likely to drive the market during the forecast period. Development of advanced testing technologies will play a critical role in early detection, precise diagnosis, and effective management of hematologic cancers, ultimately improving patient outcomes.
Increase in number of private diagnostic centers is expected to drive the global onco-hematology molecular testing industry in the next few years. Private diagnostic centers provide specialized diagnostic services, including onco-hematology molecular testing, to patients in a convenient and efficient manner.
Surge in demand for advanced and specialized healthcare services is ascribed to the rise in private diagnostic centers. Private centers often offer state-of-the-art facilities, advanced testing technologies, and personalized care, attracting patients who seek high-quality diagnostic services. These centers are equipped with sophisticated laboratory infrastructure and have skilled professionals who specialize in onco-hematology molecular testing.
Private diagnostic centers provide several advantages over traditional hospital-based laboratories. These offer shorter waiting times for appointments, quicker turnaround times for test results, and a more patient-centric approach. Patients appreciate the convenience, accessibility, and personalized attention they receive at private diagnostic centers.
Increase in number of private diagnostic centers dedicated to onco-hematology molecular testing is expected to augment the market in the near future. These centers contribute to the expansion of the market by increasing accessibility and availability of onco-hematology molecular testing services to a wider population.
Private centers often invest in advanced technologies and stay updated with the latest advancements in the field, which enhances the accuracy and efficiency of onco-hematology molecular testing.
In conclusion, rise in number of private diagnostic centers is likely to broaden onco-hematology molecular testing market outlook. Increase in access and advanced services offered by private centers contribute to improved patient outcomes and drive the demand for onco-hematology molecular testing.
In terms of blood cancer type, the acute lymphoblastic leukemia (ALL) segment accounted for the largest global onco-hematology molecular testing market share in 2022. The segment is projected to lead the global market during the forecast period.
Acute lymphoblastic leukemia is a type of blood cancer characterized by the rapid production of immature lymphocytes, affecting both children and adults. ALL is the most common type of leukemia in children, comprising a significant portion of pediatric hematologic malignancies. Early detection and accurate diagnosis of ALL are crucial for initiating timely treatment and improving survival rates in pediatric patients.
Advancements in onco-hematology molecular testing technologies have significantly enhanced the detection and monitoring of ALL. Molecular testing techniques, such as fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR), have enabled the identification of specific genetic abnormalities and chromosomal rearrangements associated with ALL. These tests provide valuable information for risk stratification, treatment selection, and disease monitoring.
The ALL segment has witnessed substantial research & development activities focused on the development of novel diagnostic markers and targeted therapies. Identification of specific genetic mutations, such as Philadelphia chromosome and fusion genes like BCR-ABL1, has led to the development of targeted therapies that have revolutionized the management of ALL.
Based on technology, the next-generation sequencing (NGS) segment is anticipated to grow at a rapid pace during the forecast period. NGS is a high-throughput DNA sequencing technology that enables simultaneous analysis of multiple genes and genomic regions, providing comprehensive insights into the genetic landscape of hematological malignancies.
NGS offers several advantages over traditional sequencing methods, such as Sanger sequencing. It allows for the detection of a range of genetic alterations, including single nucleotide variants, insertions/deletions, copy number variations, and structural rearrangements.
This comprehensive profiling capability of NGS is particularly valuable in onco-hematology molecular testing, as it enables identification of specific genetic mutations and alterations associated with different types of blood cancers.
Rise in adoption of NGS in clinical laboratories & research settings is driving the segment. NGS enables simultaneous analysis of a large number of genes, reducing the time and cost associated with conducting multiple tests individually. This efficiency makes NGS an attractive option for onco-hematology molecular testing, allowing for more streamlined and comprehensive diagnostic workflows.
Ongoing advancements in NGS technologies, such as improved sequencing platforms, enhanced bioinformatics tools, and decreased costs, contribute to the segment's rapid growth. These advancements have made NGS more accessible and affordable, allowing a wider range of laboratories and healthcare providers to incorporate this technology into their diagnostic practices.
In terms of end-user, the national reference lab/specialty lab segment is projected to account for major share of the global onco-hematology molecular testing market during the forecast period.
National reference labs and specialty labs are dedicated facilities equipped with advanced technologies and expertise to perform specialized diagnostic tests, including onco-hematology molecular testing.
National reference labs often serve as centralized facilities that receive samples from various healthcare providers and institutions. These have the capacity to handle a large volume of tests and offer standardized testing protocols, ensuring consistent and accurate results. This makes them an attractive choice for healthcare providers seeking reliable onco-hematology molecular testing services.
Specialty labs focus on specific areas, such as hematology and oncology, and have specialized expertise in conducting complex tests related to blood cancers. These have dedicated teams of pathologists, scientists, and technicians who are well-versed in the intricacies of onco-hematology molecular testing. Their specialized knowledge and experience enable them to provide comprehensive and accurate diagnostic services, contributing to the segment's market share.
National reference labs and specialty labs often have access to advanced testing technologies, including next-generation sequencing (NGS), flow cytometry, and cytogenetics. These labs continuously invest in upgrading their infrastructure and adopting state-of-the-art equipment to stay at the forefront of onco-hematology molecular testing.
According to the latest market forecast, North America is projected to account for major share of the global industry during the forecast period. The U.S. is the largest contributor to market revenue in the region.
North America is witnessing significant onco-hematology molecular testing market demand and is a key revenue contributor globally. The region's dominance can be ascribed to high incidence of hematologic cancer, aging population, rise in awareness about advanced treatment methods, and strong presence of industry players.
Surge in prevalence of leukemia, lymphoma, and multiple myeloma is one of the key drivers of the market value in North America. Statistics from the Canada Cancer Society reveal that nearly 6,700 people in Canada were diagnosed with leukemia in 2021, with 4,000 cases among men and 2,700 cases among women.
According to the American Cancer Society's data for 2023, the U.S. is expected to report 59,610 new cases of leukemia and 20,380 new cases of acute myeloid leukemia (AML) in 2023. High incidence rates of blood cancers are fueling the demand for onco-hematology molecular testing in the region.
Various organizations, such as the Leukemia & Lymphoma Society (LLS), are taking initiatives to improve blood cancer care, further contributing to onco-hematology molecular testing industry growth. In 2021, LLS provided over US$ 241 Mn in grants to support more than 42,000 blood cancer patients in the U.S.
These initiatives play a crucial role in advancing research, raising awareness, and supporting patients, thereby driving the demand for onco-hematology molecular testing.
Overall, North America is experiencing significant growth in the market, driven by high incidence of hematologic cancers, proactive initiatives by organizations, and increase in awareness about advanced treatment options. The trend is expected to continue during the forecast period.
The latest onco-hematology molecular testing market research report provides profiles of leading players operating in the global industry. Asuragen, Inc., Bio-Rad Laboratories, Inc., ICON plc, Illumina, Inc., Integrated DNA Technologies, Inc. (ArcherDx, Inc.), Invivoscribe, Inc., QIAGEN N.V., Thermo Fisher Scientific, Inc., and Cepheid are the prominent players operating in the global market.
These players are focusing on merger & acquisition, strategic collaborations, and new product launches to expand presence. Moreover, these players are following the market trends to gain market share.
Each of these players has been profiled in the market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
|
Size in 2022 |
US$ 511.3 Mn |
|
Forecast (Value) in 2031 |
More than US$ 1.5 Bn |
|
Growth Rate (CAGR) |
12.5% |
|
Forecast Period |
2023-2031 |
|
Historical Data Available for |
2017-2022 |
|
Quantitative Units |
US$ Mn/Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global industry was valued at US$ 511.3 Mn in 2022
It is projected to reach more than US$ 1.5 Bn by 2031
It is expected to advance at a CAGR of 12.5% from 2023 to 2031
The acute myeloid leukemia segment accounted for the largest market share in 2022
North America is the more lucrative region for vendors
Asuragen, Inc., Bio-Rad Laboratories, Inc., ICON plc, Illumina, Inc., Integrated DNA Technologies, Inc. (ArcherDx, Inc.), Invivoscribe, Inc., QIAGEN N.V., Thermo Fisher Scientific, Inc., and Cepheid are the prominent players operating in the global market.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Onco-hematology Molecular Testing Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Onco-hematology Molecular Testing Market Analysis and Forecast, 2017-2031
5. Key Insights
5.1. Overview of Onco-hematology Molecular Testing Market
5.2. Key Product/ Brand Analysis
5.3. Pipeline Analysis
5.4. COVID-19 Pandemic Impact on Industry
6. Global Onco-hematology Molecular Testing Market Analysis and Forecast, by Blood Cancer Type
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Blood Cancer Type, 2017-2031
6.3.1. Chronic Myeloid Leukemia
6.3.1.1. Polycythemia Vera
6.3.1.2. Essential Thrombocythaemia
6.3.1.3. Myelofibrosis
6.3.2. Myeloproliferative Neoplasms
6.3.3. Acute Myeloid Leukemia
6.3.4. Acute Lymphoblastic Leukemia
6.4. Market Attractiveness Analysis, by Blood Cancer Type
7. Global Onco-hematology Molecular Testing Market Analysis and Forecast, by Blood Cancer Biomarker
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Blood Cancer Biomarker, 2017-2031
7.3.1. BCR-ABL1 MBCR
7.3.2. JAK2
7.3.3. CALR
7.3.4. MPL
7.3.5. PML-RARA
7.3.6. NPM1
7.3.7. RUNX1-RUNX1T1
7.3.8. CBFB-MYH11
7.3.9. BCR-ABL1 mbcr
7.4. Market Attractiveness Analysis, by Blood Cancer Biomarker
8. Global Onco-hematology Molecular Testing Market Analysis and Forecast, by Technology
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by Technology, 2017-2031
8.3.1. qPCR
8.3.2. dPCR
8.3.3. Next-Generation Sequencing
8.3.4. Others
8.4. Market Attractiveness Analysis, by Technology
9. Global Onco-hematology Molecular Testing Market Analysis and Forecast, by End-user
9.1. Introduction & Definition
9.2. Key Findings/Developments
9.3. Market Value Forecast, by End-user, 2017-2031
9.3.1. National Reference Lab/Specialty Lab
9.3.2. University Hospital/Oncology Center
9.3.3. Community Hospital/IDN (Regional/National
9.4. Market Attractiveness Analysis, by End-user
10. Global Onco-hematology Molecular Testing Market Analysis and Forecast, by Region
10.1. Key Findings
10.2. Market Value Forecast, by Region, 2017-2031
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Market Attractiveness Analysis, by Region
11. North America Onco-hematology Molecular Testing Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Blood Cancer Type, 2017-2031
11.2.1. Chronic Myeloid Leukemia
11.2.1.1. Polycythemia Vera
11.2.1.2. Essential Thrombocythaemia
11.2.1.3. Myelofibrosis
11.2.2. Myeloproliferative Neoplasms
11.2.3. Acute Myeloid Leukemia
11.2.4. Acute Lymphoblastic Leukemia
11.3. Market Value Forecast, by Blood Cancer Biomarker, 2017-2031
11.3.1. BCR-ABL1 MBCR
11.3.2. JAK2
11.3.3. CALR
11.3.4. MPL
11.3.5. PML-RARA
11.3.6. NPM1
11.3.7. RUNX1-RUNX1T1
11.3.8. CBFB-MYH11
11.3.9. BCR-ABL1 mbcr
11.4. Market Value Forecast, by Technology, 2017-2031
11.4.1. qPCR
11.4.2. dPCR
11.4.3. Next-Generation Sequencing
11.4.4. Others
11.5. Market Value Forecast, by End-user, 2017-2031
11.5.1. National Reference Lab/Specialty Lab
11.5.2. University Hospital/Oncology Center
11.5.3. Community Hospital/IDN (Regional/National
11.6. Market Value Forecast, by Country, 2017-2031
11.6.1. U.S.
11.6.2. Canada
11.7. Market Attractiveness Analysis
11.7.1. By Blood Cancer Type
11.7.2. By Blood Cancer Biomarker
11.7.3. By Technology
11.7.4. By End-user
11.7.5. By Country
12. Europe Onco-hematology Molecular Testing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Blood Cancer Type, 2017-2031
12.2.1. Chronic Myeloid Leukemia
12.2.1.1. Polycythemia Vera
12.2.1.2. Essential Thrombocythaemia
12.2.1.3. Myelofibrosis
12.2.2. Myeloproliferative Neoplasms
12.2.3. Acute Myeloid Leukemia
12.2.4. Acute Lymphoblastic Leukemia
12.3. Market Value Forecast, by Blood Cancer Biomarker, 2017-2031
12.3.1. BCR-ABL1 MBCR
12.3.2. JAK2
12.3.3. CALR
12.3.4. MPL
12.3.5. PML-RARA
12.3.6. NPM1
12.3.7. RUNX1-RUNX1T1
12.3.8. CBFB-MYH11
12.3.9. BCR-ABL1 mbcr
12.4. Market Value Forecast, by Technology, 2017-2031
12.4.1. qPCR
12.4.2. dPCR
12.4.3. Next-Generation Sequencing
12.4.4. Others
12.5. Market Value Forecast, by End-user, 2017-2031
12.5.1. National Reference Lab/Specialty Lab
12.5.2. University Hospital/Oncology Center
12.5.3. Community Hospital/IDN (Regional/National
12.6. Market Value Forecast, by Country/Sub-region, 2017-2031
12.6.1. Germany
12.6.2. U.K.
12.6.3. France
12.6.4. Italy
12.6.5. Spain
12.6.6. Rest of Europe
12.7. Market Attractiveness Analysis
12.7.1. By Blood Cancer Type
12.7.2. By Blood Cancer Biomarker
12.7.3. By Technology
12.7.4. By End-user
12.7.5. By Country/Sub-region
13. Asia Pacific Onco-hematology Molecular Testing Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Blood Cancer Type, 2017-2031
13.2.1. Chronic Myeloid Leukemia
13.2.1.1. Polycythemia Vera
13.2.1.2. Essential Thrombocythaemia
13.2.1.3. Myelofibrosis
13.2.2. Myeloproliferative Neoplasms
13.2.3. Acute Myeloid Leukemia
13.2.4. Acute Lymphoblastic Leukemia
13.3. Market Value Forecast, by Blood Cancer Biomarker, 2017-2031
13.3.1. BCR-ABL1 MBCR
13.3.2. JAK2
13.3.3. CALR
13.3.4. MPL
13.3.5. PML-RARA
13.3.6. NPM1
13.3.7. RUNX1-RUNX1T1
13.3.8. CBFB-MYH11
13.3.9. BCR-ABL1 mbcr
13.4. Market Value Forecast, by Technology, 2017-2031
13.4.1. qPCR
13.4.2. dPCR
13.4.3. Next-Generation Sequencing
13.4.4. Others
13.5. Market Value Forecast, by End-user, 2017-2031
13.5.1. National Reference Lab/Specialty Lab
13.5.2. University Hospital/Oncology Center
13.5.3. Community Hospital/IDN (Regional/National
13.6. Market Value Forecast, by Country/Sub-region, 2017-2031
13.6.1. China
13.6.2. Japan
13.6.3. India
13.6.4. Australia & New Zealand
13.6.5. Rest of Asia Pacific
13.7. Market Attractiveness Analysis
13.7.1. By Blood Cancer Type
13.7.2. By Blood Cancer Biomarker
13.7.3. By Technology
13.7.4. By End-user
13.7.5. By Country/Sub-region
14. Latin America Onco-hematology Molecular Testing Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Blood Cancer Type, 2017-2031
14.2.1. Chronic Myeloid Leukemia
14.2.1.1. Polycythemia Vera
14.2.1.2. Essential Thrombocythaemia
14.2.1.3. Myelofibrosis
14.2.2. Myeloproliferative Neoplasms
14.2.3. Acute Myeloid Leukemia
14.2.4. Acute Lymphoblastic Leukemia
14.3. Market Value Forecast, by Blood Cancer Biomarker, 2017-2031
14.3.1. BCR-ABL1 MBCR
14.3.2. JAK2
14.3.3. CALR
14.3.4. MPL
14.3.5. PML-RARA
14.3.6. NPM1
14.3.7. RUNX1-RUNX1T1
14.3.8. CBFB-MYH11
14.3.9. BCR-ABL1 mbcr
14.4. Market Value Forecast, by Technology, 2017-2031
14.4.1. qPCR
14.4.2. dPCR
14.4.3. Next-Generation Sequencing
14.4.4. Others
14.5. Market Value Forecast, by End-user, 2017-2031
14.5.1. National Reference Lab/Specialty Lab
14.5.2. University Hospital/Oncology Center
14.5.3. Community Hospital/IDN (Regional/National
14.6. Market Value Forecast, by Country/Sub-region, 2017-2031
14.6.1. Brazil
14.6.2. Mexico
14.6.3. Rest of Latin America
14.7. Market Attractiveness Analysis
14.7.1. By Blood Cancer Type
14.7.2. By Blood Cancer Biomarker
14.7.3. By Technology
14.7.4. By End-user
14.7.5. By Country/Sub-region
15. Middle East & Africa Onco-hematology Molecular Testing Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Blood Cancer Type, 2017-2031
15.2.1. Chronic Myeloid Leukemia
15.2.1.1. Polycythemia Vera
15.2.1.2. Essential Thrombocythaemia
15.2.1.3. Myelofibrosis
15.2.2. Myeloproliferative Neoplasms
15.2.3. Acute Myeloid Leukemia
15.2.4. Acute Lymphoblastic Leukemia
15.3. Market Value Forecast, by Blood Cancer Biomarker, 2017-2031
15.3.1. BCR-ABL1 MBCR
15.3.2. JAK2
15.3.3. CALR
15.3.4. MPL
15.3.5. PML-RARA
15.3.6. NPM1
15.3.7. RUNX1-RUNX1T1
15.3.8. CBFB-MYH11
15.3.9. BCR-ABL1 mbcr
15.4. Market Value Forecast, by Technology, 2017-2031
15.4.1. qPCR
15.4.2. dPCR
15.4.3. Next-Generation Sequencing
15.4.4. Others
15.5. Market Value Forecast, by End-user, 2017-2031
15.5.1. National Reference Lab/Specialty Lab
15.5.2. University Hospital/Oncology Center
15.5.3. Community Hospital/IDN (Regional/National
15.6. Market Value Forecast, by Country/Sub-region, 2017-2031
15.6.1. GCC Countries
15.6.2. South Africa
15.6.3. Rest of Middle East & Africa
15.7. Market Attractiveness Analysis
15.7.1. By Blood Cancer Type
15.7.2. By Blood Cancer Biomarker
15.7.3. By Technology
15.7.4. By End-user
15.7.5. By Country/Sub-region
16. Competition Landscape
16.1. Market Player - Competition Matrix (by tier and size of companies)
16.2. Market Share Analysis, by Company, 2022
16.3. Company Profiles
16.3.1. Asuragen, Inc.
16.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.1.2. Product Portfolio
16.3.1.3. Financial Overview
16.3.1.4. SWOT Analysis
16.3.1.5. Strategic Overview
16.3.2. Bio-Rad Laboratories, Inc.
16.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.2.2. Product Portfolio
16.3.2.3. Financial Overview
16.3.2.4. SWOT Analysis
16.3.2.5. Strategic Overview
16.3.3. ICON plc
16.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.3.2. Product Portfolio
16.3.3.3. Financial Overview
16.3.3.4. SWOT Analysis
16.3.3.5. Strategic Overview
16.3.4. Integrated DNA Technologies, Inc. (ArcherDx, Inc.)
16.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.4.2. Product Portfolio
16.3.4.3. Financial Overview
16.3.4.4. SWOT Analysis
16.3.4.5. Strategic Overview
16.3.5. Invivoscribe, Inc.
16.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.5.2. Product Portfolio
16.3.5.3. Financial Overview
16.3.5.4. SWOT Analysis
16.3.5.5. Strategic Overview
16.3.6. QIAGEN N.V.
16.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.6.2. Product Portfolio
16.3.6.3. Financial Overview
16.3.6.4. SWOT Analysis
16.3.6.5. Strategic Overview
16.3.7. Thermo Fisher Scientific, Inc.
16.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.7.2. Product Portfolio
16.3.7.3. Financial Overview
16.3.7.4. SWOT Analysis
16.3.7.5. Strategic Overview
16.3.8. Cepheid
16.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.8.2. Product Portfolio
16.3.8.3. Financial Overview
16.3.8.4. SWOT Analysis
16.3.8.5. Strategic Overview
16.3.9. Illumina, Inc.
16.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.9.2. Product Portfolio
16.3.9.3. Financial Overview
16.3.9.4. SWOT Analysis
16.3.9.5. Strategic Overview
List of Tables
Table 01: Global Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Type, 2017-2031
Table 02: Global Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Biomarker, 2017‒2031
Table 03: Global Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Technology, 2017-2031
Table 04: Global Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 05: Global Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Region, 2017-2031
Table 06: North America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Country, 2017-2031
Table 07: North America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Type, 2017-2031
Table 08: North America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Biomarker, 2017‒2031
Table 09: North America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Technology, 2017-2031
Table 10: North America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 11: Europe Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 12: Europe Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Type, 2017-2031
Table 13: Europe Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Biomarker, 2017‒2031
Table 14: Europe Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Technology, 2017-2031
Table 15: Europe Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 16: Asia Pacific Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 17: Asia Pacific Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Type, 2017-2031
Table 18: Asia Pacific Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Biomarker, 2017‒2031
Table 19: Asia Pacific Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Technology, 2017-2031
Table 20: Asia Pacific Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 21: Latin America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 22: Latin America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Type, 2017-2031
Table 23: Latin America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Biomarker, 2017‒2031
Table 24: Latin America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Technology, 2017-2031
Table 25: Latin America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 26: Middle East & Africa Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 27: Middle East & Africa Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Type, 2017-2031
Table 28: Middle East & Africa Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Blood Cancer Biomarker, 2017‒2031
Table 29: Middle East & Africa Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by Technology, 2017-2031
Table 30: Middle East & Africa Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
List of Figures
Figure 01: Global Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, 2017-2031
Figure 02: Global Onco-hematology Molecular Testing Market Value Share, by Blood Cancer Type, 2022
Figure 03: Global Onco-hematology Molecular Testing Market Value Share, by Blood Cancer Biomarker, 2022
Figure 04: Global Onco-hematology Molecular Testing Market Value Share, by Technology, 2022
Figure 05: Global Onco-hematology Molecular Testing Market Value Share, by End-user, 2022
Figure 06: Global Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Type, 2022 and 2031
Figure 07: Global Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Type, 2023-2031
Figure 08: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by Chronic Myeloid Leukemia , 2017-2031
Figure 09: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by Myeloproliferative Neoplasms, 2017-2031
Figure 10: Global Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Biomarker, 2022 and 2031
Figure 11: Global Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Biomarker 2023-2031
Figure 12: Global Onco-hematology Molecular Testing Market Value (US$ Mn), by BCR-ABL1 MBCR, 2017‒2031
Figure 13: Global Onco-hematology Molecular Testing Market Value (US$ Mn), by JAK2, 2017‒2031
Figure 14: Global Onco-hematology Molecular Testing Market Value (US$ Mn), by Irreversible CALR, 2017‒2031
Figure 15: Global Onco-hematology Molecular Testing Market Value (US$ Mn), by MPL, 2017‒2031
Figure 16: Global Onco-hematology Molecular Testing Market Value (US$ Mn), by Others, 2017‒2031
Figure 17: Global Onco-hematology Molecular Testing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 18: Global Onco-hematology Molecular Testing Market Attractiveness Analysis, Technology, 2023-2031
Figure 19: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by QPCR , 2017-2031
Figure 20: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by DPCR, 2017-2031
Figure 21: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by Next-Generation Sequencing, 2017-2031
Figure 22: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by Others, 2017-2031
Figure 23: Global Onco-hematology Molecular Testing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 24: Global Onco-hematology Molecular Testing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 25: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by National Reference Lab/Specialty Lab, 2017-2031
Figure 26: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by University Hospital/Oncology Center, 2017-2031
Figure 27: Global Onco-hematology Molecular Testing Market Revenue (US$ Mn), by Others, 2017-2031
Figure 28: Global Onco-hematology Molecular Testing Market Value Share Analysis, by Region, 2022 and 2031
Figure 29: Global Onco-hematology Molecular Testing Market Attractiveness Analysis, by Region, 2023-2031
Figure 30: North America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, 2017-2031
Figure 31: North America Onco-hematology Molecular Testing Market Value Share Analysis, by Country, 2022 and 2031
Figure 32: North America Onco-hematology Molecular Testing Market Attractiveness Analysis, by Country, 2023-2031
Figure 33: North America Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Type, 2022 and 2031
Figure 34: North America Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Type, 2023-2031
Figure 35: North America Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Biomarker, 2022 and 2031
Figure 36: North America Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Biomarker 2023-2031
Figure 37: North America Onco-hematology Molecular Testing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 38: North America Onco-hematology Molecular Testing Market Attractiveness Analysis, Technology, 2023-2031
Figure 39: North America Onco-hematology Molecular Testing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 40: North America Onco-hematology Molecular Testing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 41: Europe Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, 2017-2031
Figure 42: Europe Onco-hematology Molecular Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 43: Europe Onco-hematology Molecular Testing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 44: Europe Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Type, 2022 and 2031
Figure 45: Europe Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Type, 2023-2031
Figure 46: Europe Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Biomarker, 2022 and 2031
Figure 47: Europe Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Biomarker 2023-2031
Figure 48: Europe Onco-hematology Molecular Testing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 49: Europe Onco-hematology Molecular Testing Market Attractiveness Analysis, Technology, 2023-2031
Figure 50: Europe Onco-hematology Molecular Testing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 51: Europe Onco-hematology Molecular Testing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 52: Asia Pacific Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, 2017-2031
Figure 53: Asia Pacific Onco-hematology Molecular Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 54: Asia Pacific Onco-hematology Molecular Testing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 55: Asia Pacific Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Type, 2022 and 2031
Figure 56: Asia Pacific Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Type, 2023-2031
Figure 57: Asia Pacific Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Biomarker, 2022 and 2031
Figure 58: Asia Pacific Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Biomarker 2023-2031
Figure 59: Asia Pacific Onco-hematology Molecular Testing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 60: Asia Pacific Onco-hematology Molecular Testing Market Attractiveness Analysis, Technology, 2023-2031
Figure 61: Asia Pacific Onco-hematology Molecular Testing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 62: Asia Pacific Onco-hematology Molecular Testing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 63: Latin America Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, 2017-2031
Figure 64: Latin America Onco-hematology Molecular Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 65: Latin America Onco-hematology Molecular Testing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 66: Latin America Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Type, 2022 and 2031
Figure 67: Latin America Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Type, 2023-2031
Figure 68: Latin America Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Biomarker, 2022 and 2031
Figure 69: Latin America Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Biomarker, 2023-2031
Figure 70: Latin America Onco-hematology Molecular Testing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 71: Latin America Onco-hematology Molecular Testing Market Attractiveness Analysis, Technology, 2023-2031
Figure 72: Latin America Onco-hematology Molecular Testing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 73: Latin America Onco-hematology Molecular Testing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 74: Middle East & Africa Onco-hematology Molecular Testing Market Value (US$ Mn) Forecast, 2017-2031
Figure 75: Middle East & Africa Onco-hematology Molecular Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 76: Middle East & Africa Onco-hematology Molecular Testing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 77: Middle East & Africa Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Type, 2022 and 2031
Figure 78: Middle East & Africa Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Type, 2023-2031
Figure 79: Middle East & Africa Onco-hematology Molecular Testing Market Value Share Analysis, by Blood Cancer Biomarker, 2022 and 2031
Figure 80: Middle East & Africa Onco-hematology Molecular Testing Market Attractiveness Analysis, by Blood Cancer Biomarker 2023-2031
Figure 81: Middle East & Africa Onco-hematology Molecular Testing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 82: Middle East & Africa Onco-hematology Molecular Testing Market Attractiveness Analysis, by Technology, 2023-2031
Figure 83: Middle East & Africa Onco-hematology Molecular Testing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 84: Middle East & Africa Onco-hematology Molecular Testing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 85: Global Onco-hematology Molecular Testing Market Share Analysis, by Company (2022)