Reports
Reports
The medical device contract manufacturing market is relied upon to show extensive development because of variables, for example, expanding medical problems and the requirement for appropriate determination. Besides, the flare-up of the COVID-19 has attacked the world and the interest in testing units has shown a generous ascent. This pandemic has assumed an imperative part in speeding up the development of the medical device contract manufacturing market.
The agreement fabricating area helps with offering support in various limits like plan and creation. It assists with keeping up with center-around-center abilities and key preparation. The rising interest in clinical gadgets in the pandemic situation has shown vigorous development in the medical device contract manufacturing market. The developing interest for clinical hardware has likewise brought about the requirement for organizations producing clinical gadgets, which thusly, is helping the development of the medical device contract manufacturing market.
The clinical gadget fabricating industry is quickly developing across the globe and alongside that agreement producing administrations structure an enormous piece of the business. Throughout the last numerous many years, the agreement fabricating area alongside the clinical gadget producing industry has confronted the difficulties of satisfying the spiraling needs and has reshaped clinical innovation. Indeed, even presently, when COVID-19 is desolating the world, there is a deficiency of clinical units for testing the presence of an infection in a person. Worldwide pandemics, for example, COVID-19 alongside the typical expanding interest for clinical gadgets are probably going to reinforce the development of the worldwide medical device contract manufacturing market sooner rather than later. With the rising interest in clinical gadgets, it is normal that the requirement for individuals and organizations fabricating clinical gadgets will likewise rise. Accordingly, it is normal that agreement assembling will play a critical in the years to come.
The key elements driving the development of the medical device contract manufacturing market incorporates the general development of the clinical gadgets market, essentially because of rising infection pervasiveness, future, and the geriatric populace. Innovative progression has incited end clients to redesign or refresh their assembling frameworks. As this is an expensive interaction, they hope to contract fabricating. Furthermore, the COVID-19 flare-up has sped up the reception of cutting-edge diagnostics and patient consideration gadgets for better treatment of the executives. Nevertheless, market development is blocked by the developing combination in the clinical gadgets market. Bigger players, to foster their assembling capacities to save costs, center on the securing of more modest players and CMOs themselves.
The global medical device market is classified on the basis of device type, services type, type, class of device, and region. In terms of device type, the market is categorized into IVD devices, diagnostic imaging devices, cardiovascular devices, drug delivery devices, orthopedic devices, ophthalmic devices, ophthalmology devices, diabetes care devices, dental devices, endoscopy devices, respiratory care devices, surgical devices, gynecology or urology devices, personal care, neurology devices, and other devices. Among these, the drug delivery devices, and IVD devices segment are further sub-categorized. Based on type, the market is grouped into other packaging and assembling services, labeling, primary & secondary packaging, and other services. Further classification of class of device segment includes Classes I, II, and III. The gadget development and manufacturing administrations fragment overwhelmed this market. The expanding reception of agreement manufacturing administrations in the medical gadget industry, development in the medical devices market (particularly in the single-utilize dispensable medical devices market), and further developing gadget development and manufacturing abilities are the main considerations liable for the huge portion of this section.
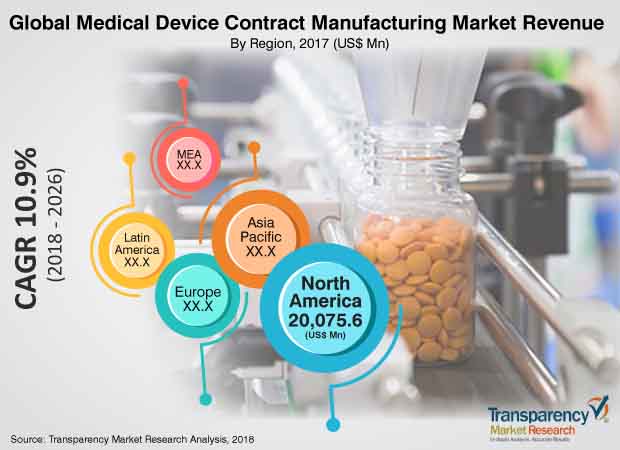
North America overwhelmed the worldwide medical gadget contract manufacturing market in 2017, inferable from a profoundly evolved medical services area, expansion in mindfulness among medical care suppliers about medical devices, and constant development of cutting edge medical gadget items. North America is likewise an alluring district of the market, as far as income. The ascent in the quantity of government drives and laws and expansion in populace drive the medical gadget contract manufacturing market in the area. Innovative progressions and an increment in manufacturing locales are relied upon to drive the medical gadget contract manufacturing market in the Asia Pacific, Latin America, and Middle East and Africa during the conjecture time frame.
Medical device contract manufacturing market to reach US$ 118.9 Bn by 2026
Medical device contract manufacturing market is projected to expand at a CAGR of 10.9% from 2018 to 2026
Medical device contract manufacturing market is driven by increase in the number of cases of various disorders across the globe
North America and Europe are projected to dominate the global medical devices contract manufacturing market, owing to a highly developed health care sector
Key players in the global medical devices contract manufacturing market include Flextronics International, LTD., Jabil Inc., Benchmark Electronics, Inc., Integer Holdings Corporation (Greatbatch), West Pharmaceutical Services, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary : Global Medical Device Contract Manufacturing Market
4. Market Overview
4.1. Introduction
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Medical Device Contract Manufacturing Market Analysis and Forecasts, 2016–2026
5. Key Insights
5.1. Regulatory Scenario by Region
5.2. Selection Criteria for Medical Device Contract Manufacturing
5.3. Supply Chain Analysis
6. Global Medical Device Contract Manufacturing Market Analysis and Forecasts, by Device Type
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Device Type, 2016–2026
6.3.1. In-vitro Diagnostic Medical Devices
6.3.2. Diagnostic Imaging & Medical Equipment
6.3.3. Drug Delivery Devices
6.3.4. Patient Monitoring Devices
6.3.5. Minimally Access Surgical Instruments
6.3.6. Therapeutic Patient Assistive Devices
6.3.7. Others
6.4. Market Attractiveness, by Device Type
7. Global Medical Device Contract Manufacturing Market Analysis and Forecasts, by Type of Manufacturing
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Type of Manufacturing, 2016–2026
7.3.1. Raw Materials
7.3.2. Electronics
7.3.3. Finished Goods
7.4. Market Attractiveness, by Type of Manufacturing
8. Global Medical Device Contract Manufacturing Market Analysis and Forecasts, by Services
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Services , 2016–2026
8.3.1. Prototype Development
8.3.2. Finished Device Manufacturing
8.3.3. Assembly & Packaging
8.3.4. Testing & Regulatory Support Services
8.3.5. Molding & Casting
8.3.6. Others
8.4. Market Attractiveness, by Service
9. Global Medical Device Contract Manufacturing Market Analysis and Forecasts, by Application
9.1. Introduction & Definition
9.2. Key Findings/Developments
9.3. Market Value Forecast, by Application, 2016–2026
9.3.1. Cardiovascular
9.3.2. Orthopedic
9.3.3. Neurovascular
9.3.4. Pulmonary
9.3.5. Oncology
9.3.6. Laparoscopy
9.3.7. Urology & Gynecology
9.3.8. Radiology
9.3.9. Others
9.4. Market Attractiveness, by Application
10. Global Medical Device Contract Manufacturing Market Analysis and Forecasts, by Region
10.1. Key Findings
10.2. Market Value Forecast, by Region
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Market Attractiveness, by Country/Region
11. North America Medical Device Contract Manufacturing Market Analysis and Forecast
11.1.Introduction
11.2.Market Value Forecast, by Device Type, 2016–2026
11.2.1. In-vitro Diagnostic Medical Devices
11.2.2. Diagnostic Imaging & Medical Equipment
11.2.3. Drug Delivery Devices
11.2.4. Patient Monitoring Devices
11.2.5. Minimally Access Surgical Instruments
11.2.6. Therapeutic Patient Assistive Devices
11.2.7. Others
11.3.Market Value Forecast, by Type of Manufacturing, 2016–2026
11.3.1. Raw Materials
11.3.2. Electronics
11.3.3. Finished Goods
11.4.Market Value Forecast, by Services , 2016–2026
11.4.1. Prototype Development
11.4.2. Finished Device Manufacturing
11.4.3. Assembly & Packaging
11.4.4. Testing & Regulatory Support Services
11.4.5. Molding & Casting
11.4.6. Others
11.5.Market Value Forecast, by Application, 2016–2026
11.5.1. Cardiovascular
11.5.2. Orthopedic
11.5.3. Neurovascular
11.5.4. Pulmonary
11.5.5. Oncology
11.5.6. Laparoscopy
11.5.7. Urology & Gynecology
11.5.8. Radiology
11.5.9. Others
11.6.Market Value Forecast, by Country, 2016–2026
11.6.1. U.S.
11.6.2. Canada
11.7.Market Attractiveness Analysis
11.7.1. By Device Type
11.7.2. By Type of Manufacturing
11.7.3. By Services
11.7.4. By Application
11.7.5. By Country
12. Europe Medical Device Contract Manufacturing Market Analysis and Forecast
12.1.Introduction
12.2.Market Value Forecast, by Device Type, 2016–2026
12.2.1. In-vitro Diagnostic Medical Devices
12.2.2. Diagnostic Imaging & Medical Equipment
12.2.3. Drug Delivery Devices
12.2.4. Patient Monitoring Devices
12.2.5. Minimally Access Surgical Instruments
12.2.6. Therapeutic Patient Assistive Devices
12.2.7. Others
12.3.Market Value Forecast, by Type of Manufacturing, 2016–2026
12.3.1. Raw Materials
12.3.2. Electronics
12.3.3. Finished Goods
12.4.Market Value Forecast, by Service , 2016–2026
12.4.1. Prototype Development
12.4.2. Finished Device Manufacturing
12.4.3. Assembly & Packaging
12.4.4. Testing & Regulatory Support Services
12.4.5. Molding & Casting
12.4.6. Others
12.5.Market Value Forecast, by Application, 2016–2026
12.5.1. Cardiovascular
12.5.2. Orthopedic
12.5.3. Neurovascular
12.5.4. Pulmonary
12.5.5. Oncology
12.5.6. Laparoscopy
12.5.7. Urology & Gynecology
12.5.8. Radiology
12.5.9. Others
12.6.Market Value Forecast, by Country, 2016–2026
12.6.1. Germany
12.6.2. U.K.
12.6.3. France
12.6.4. Spain
12.6.5. Italy
12.6.6. Rest of Europe
12.7.Market Attractiveness Analysis
12.7.1. By Device Type
12.7.2. By Type of Manufacturing
12.7.3. By Services
12.7.4. By Application
12.7.5. By Country
13. Asia Pacific Medical Device Contract Manufacturing Market Analysis and Forecast
13.1.Introduction
13.2.Market Value Forecast, by Device Type, 2016–2026
13.2.1. In-vitro Diagnostic Medical Devices
13.2.2. Diagnostic Imaging & Medical Equipment
13.2.3. Drug Delivery Devices
13.2.4. Patient Monitoring Devices
13.2.5. Minimally Access Surgical Instruments
13.2.6. Therapeutic Patient Assistive Devices
13.2.7. Others
13.3.Market Value Forecast, by Type of Manufacturing, 2016–2026
13.3.1. Raw Materials
13.3.2. Electronics
13.3.3. Finished Goods
13.4.Market Value Forecast, by Services , 2016–2026
13.4.1. Prototype Development
13.4.2. Finished Device Manufacturing
13.4.3. Assembly & Packaging
13.4.4. Testing & Regulatory Support Services
13.4.5. Molding & Casting
13.4.6. Others
13.5.Market Value Forecast, by Application, 2016–2026
13.5.1. Cardiovascular
13.5.2. Orthopedic
13.5.3. Neurovascular
13.5.4. Pulmonary
13.5.5. Oncology
13.5.6. Laparoscopy
13.5.7. Urology & Gynecology
13.5.8. Radiology
13.5.9. Others
13.6.Market Value Forecast, by Country, 2016–2026
13.6.1. China
13.6.2. Japan
13.6.3. India
13.6.4. Australia & New Zealand
13.6.5. Rest of Asia Pacific
13.7.Market Attractiveness Analysis
13.7.1. By by Device Type
13.7.2. By by Type of Manufacturing
13.7.3. By by Services
13.7.4. By by Application
13.7.5. By by Country
14. Latin America Medical Device Contract Manufacturing Market Analysis and Forecast
14.1.Introduction
14.2.Market Value Forecast, by Device Type, 2016–2026
14.2.1. In-vitro Diagnostic Medical Devices
14.2.2. Diagnostic Imaging & Medical Equipment
14.2.3. Drug Delivery Devices
14.2.4. Patient Monitoring Devices
14.2.5. Minimally Access Surgical Instruments
14.2.6. Therapeutic Patient Assistive Devices
14.2.7. Others
14.3.Market Value Forecast, by Type of Manufacturing, 2016–2026
14.3.1. Raw Materials
14.3.2. Electronics
14.3.3. Finished Goods
14.4.Market Value Forecast, by Services , 2016–2026
14.4.1. Prototype Development
14.4.2. Finished Device Manufacturing
14.4.3. Assembly & Packaging
14.4.4. Testing & Regulatory Support Services
14.4.5. Molding & Casting
14.4.6. Others
14.5.Market Value Forecast, by Application, 2016–2026
14.5.1. Cardiovascular
14.5.2. Orthopedic
14.5.3. Neurovascular
14.5.4. Pulmonary
14.5.5. Oncology
14.5.6. Laparoscopy
14.5.7. Urology & Gynecology
14.5.8. Radiology
14.5.9. Others
14.6.Market Value Forecast, by Country, 2016–2026
14.6.1. Brazil
14.6.2. Mexico
14.6.3. Rest of Latin America
14.7.Market Attractiveness Analysis
14.7.1. By Device Type
14.7.2. By Type of Manufacturing
14.7.3. By Services
14.7.4. By Application
14.7.5. By Country
15. Middle East & Africa Medical Device Contract Manufacturing Market Analysis and Forecast
15.1.Introduction
15.2.Market Value Forecast, by Device Type, 2016–2026
15.2.1. In-vitro Diagnostic Medical Devices
15.2.2. Diagnostic Imaging & Medical Equipment
15.2.3. Drug Delivery Devices
15.2.4. Patient Monitoring Devices
15.2.5. Minimally Access Surgical Instruments
15.2.6. Therapeutic Patient Assistive Devices
15.2.7. Others
15.3.Market Value Forecast, by Type of Manufacturing, 2016–2026
15.3.1. Raw Materials
15.3.2. Electronics
15.3.3. Finished Goods
15.4.Market Value Forecast, by Services , 2016–2026
15.4.1. Prototype Development
15.4.2. Finished Device Manufacturing
15.4.3. Assembly & Packaging
15.4.4. Testing & Regulatory Support Services
15.4.5. Molding & Casting
15.4.6. Others
15.5.Market Value Forecast, by Application, 2016–2026
15.5.1. Cardiovascular
15.5.2. Orthopedic
15.5.3. Neurovascular
15.5.4. Pulmonary
15.5.5. Oncology
15.5.6. Laparoscopy
15.5.7. Urology & Gynecology
15.5.8. Radiology
15.5.9. Others
15.6.Market Value Forecast, by Country, 2016–2026
15.6.1. GCC Countries
15.6.2. South Africa
15.6.3. Rest of Middle East & Africa
15.7.Market Attractiveness Analysis
15.7.1. By Device Type
15.7.2. By Type of Manufacturing
15.7.3. By Services
15.7.4. By Application
15.7.5. By Country
16. Competition Landscape
16.1.Market Share Analysis by Company (2017)
16.2. Company Profiles
16.2.1. Flextronics International, LTD.
16.2.2. Jabil Inc.
16.2.3. Benchmark Electronics, Inc.
16.2.4. Integer Holdings Corporation (Greatbatch)
16.2.5. West Pharmaceutical Services, Inc.
16.2.6. Tecomet, Inc.
16.2.7. Nortech Systems
16.2.8. TE Connectivity (Creganna Medical)
16.2.9. Forefront Medical Technologies
16.2.10. Nordson Corporation
List of Tables
Table 01: Global Medical Device Contract Manufacturing Market Size (US$ Mn) Forecast, by Device Type, 2016–2026
Table 02: Global Medical Device Contract Manufacturing Market Size (US$ Mn) Forecast, by Type of Manufacturing , 2016–2026
Table 03: Global Medical Device Contract Manufacturing Market Size (US$ Mn) Forecast, by Service, 2016–2026
Table 04: Global Medical Device Contract Manufacturing Market Size (US$ Mn) Forecast, by Application, 2016–2026
Table 05: Global Medical Device Contract Manufacturing Market Value (US$ Mn) Forecast, by Region , 2016–2026
Table 06: North America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Country, 2016–2026
Table 07: North America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Device Type, 2016–2026
Table 08: North America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Type of Manufacturing, 2016–2026
Table 09: North America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Services, 2016–2026
Table 10: North America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Application, 2016–2026
Table 11: Europe Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 12: Europe Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Device Type, 2016–2026
Table 13: Europe Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Type of Manufacturing, 2016–2026
Table 14: Europe Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Services, 2016–2026
Table 15: Europe Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Application, 2016–2026
Table 16: Asia Pacific Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 17: Asia Pacific Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Device Type, 2016–2026
Table 18: Asia Pacific Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Type of Manufacturing, 2016–2026
Table 19: Asia Pacific Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Services, 2016–2026
Table 20: Asia Pacific Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Application, 2016–2026
Table 21: Latin America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 22: Latin America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Device Type, 2016–2026
Table 23: Latin America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Type of Manufacturing, 2016–2026
Table 24: Latin America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Services, 2016–2026
Table 25: Latin America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Application, 2016–2026
Table 26: Middle East & Africa Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Country/Sub-region, 2016–2026
Table 27: Middle East & Africa Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Type of Manufacturing, 2016–2026
Table 28: Middle East & Africa Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Services, 2016–2026
Table 29: Middle East & Africa Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Application, 2016–2026
Table 30: Middle East & Africa Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast, by Device Type, 2016–2026
List of Figures
Figure 01: Global Medical Device Contract Manufacturing Market Value (US$ Mn) Forecast, 2016–2026
Figure 02: Global Medical Device Contract Manufacturing Market Value Share Analysis, by Device Type, 2017 and 2026
Figure 03: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by In-vitro Diagnostic
Figure 04: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Diagnostic Imaging &
Figure 05: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Drug Delivery Devices, 2016–2026
Figure 06: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Patient Monitoring Devices, 2016–2026
Figure 07: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Minimally Access
Figure 08: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Therapeutic Patient
Figure 09: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Other Device Types, 2016–2026
Figure 10: Global Medical Device Contract Manufacturing Market Attractiveness Analysis, by Device Type, 2018–2026
Figure 11: Global Medical Device Contract Manufacturing Market Value Share Analysis, by Type of Manufacturing, 2018 and 2026
Figure 12: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Raw Materials, 2016-2026
Figure 13: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Electronics, 2016-2026
Figure 14: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Finished Goods, 2016-2026
Figure 15: Global medical device contract Manufacturing Market Attractiveness Analysis, by Type of Manufacturing, 2018–2026
Figure 16: Global Medical Device Contract Manufacturing Market Value Share Analysis, by Services, 2017 and 2026
Figure 17: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Prototype Development, 2016–2026
Figure 18: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Finished Device Manufacturing, 2016–2026
Figure 19: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Assembly & Packaging, 2016–2026
Figure 20: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Testing & Regulatory Support Services, 2016–2026
Figure 21: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Molding & Casting, 2016–2026
Figure 22: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Others, 2016–2026
Figure 23: Global Medical Device Contract Manufacturing Market Attractiveness Analysis, by Services, 2018–2026
Figure 24: Global Medical Device Contract Manufacturing Market Value Share Analysis, by Application, 2017 and 2026
Figure 25: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Cardiovascular, 2016–2026
Figure 26: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Orthopedic, 2016–2026
Figure 27: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Neurovascular, 2016–2026
Figure 28: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Pulmonary, 2016–2026
Figure 29: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Oncology, 2016–2026
Figure 30: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Laparoscopy, 2016–2026
Figure 31: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Urology & Gynecology, 2016–2026
Figure 32: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Radiology, 2016–2026
Figure 33: Global Medical Device Contract Manufacturing Market Revenue (US$ Mn), by Others, 2016–2026
Figure 34: Global Medical Device Contract Manufacturing Market Attractiveness Analysis, by Application, 2018–2026
Figure 35: Global Medical Device Contract Manufacturing Market Value Share Analysis, by Region, 2017 and 2026
Figure 36: Global Medical Device Contract Manufacturing Market Attractiveness Analysis, by Region
Figure 37: North America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 38: North America Medical Device Contract Manufacturing Market Value Share (%), by Country, 2017 and 2026
Figure 39: North America Medical Device Contract Manufacturing Market Attractiveness, by Country, 2018–2026
Figure 40: North America Medical Device Contract Manufacturing Market Value Share (%), by Device Type, 2017 and 2026
Figure 41: North America Medical Device Contract Manufacturing Market Attractiveness, by Device Type, 2018–2026
Figure 42: North America Medical Device Contract Manufacturing Market Value Share (%), by Type of Manufacturing, 2017 and 2026
Figure 43: North America Medical Device Contract Manufacturing Market Attractiveness, by Type of Manufacturing, 2018–2026
Figure 44: North America Medical Device Contract Manufacturing Market Value Share (%), by Services, 2017 and 2026
Figure 45: North America Medical Device Contract Manufacturing Market Attractiveness, by Service, 2018–2026
Figure 46: North America Medical Device Contract Manufacturing Market Value Share (%), by Application, 2017 and 2026
Figure 47: North America Medical Device Contract Manufacturing Market Attractiveness, by Application, 2018–2026
Figure 48: Europe Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 49: Europe Medical Device Contract Manufacturing Market Value Share (%), by Country, 2017 and 2026
Figure 50: Europe Medical Device Contract Manufacturing Market Attractiveness, by Country/Sub-region, 2018–2026
Figure 51: Europe Medical Device Contract Manufacturing Market Value Share (%), by Device Type, 2017 and 2026
Figure 52: Europe Medical Device Contract Manufacturing Market Attractiveness, by Device Type, 2018–2026
Figure 53: Europe Medical Device Contract Manufacturing Market Value Share (%), by Type of Manufacturing, 2017 and 2026
Figure 54: Europe Medical Device Contract Manufacturing Market Attractiveness, by Type of Manufacturing, 2018–2026
Figure 55: Europe Medical Device Contract Manufacturing Market Value Share (%), by Service, 2017 and 2026
Figure 56: Europe Medical Device Contract Manufacturing Market Attractiveness, by Service, 2018–2026
Figure 57: Europe Medical Device Contract Manufacturing Market Value Share (%), by Application, 2017 and 2026
Figure 58: Europe Medical Device Contract Manufacturing Market Attractiveness, by Application, 2018–2026
Figure 59: Asia Pacific Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 60: Asia Pacific Medical Device Contract Manufacturing Market Value Share (%), by Country/Sub-region, 2017 and 2026
Figure 61: Asia Pacific Medical Device Contract Manufacturing Market Attractiveness, by Country/Sub-region, 2018–2026
Figure 62: Asia Pacific Medical Device Contract Manufacturing Market Value Share (%), by Device Type, 2017 and 2026
Figure 63: Asia Pacific Medical Device Contract Manufacturing Market Attractiveness, by Device Type, 2018–2026
Figure 64: Asia Pacific Medical Device Contract Manufacturing Market Value Share (%), by Type of Manufacturing, 2017 and 2026
Figure 65: Asia Pacific Medical Device Contract Manufacturing Market Attractiveness, by Type of Manufacturing, 2018–2026
Figure 66: Asia Pacific Medical Device Contract Manufacturing Market Value Share (%), by Service, 2017 and 2026
Figure 67: Asia Pacific Medical Device Contract Manufacturing Market Attractiveness, by Service, 2018–2026
Figure 68: Asia Pacific Medical Device Contract Manufacturing Market Value Share (%), by Application, 2017 and 2026
Figure 69: Asia Pacific Medical Device Contract Manufacturing Market Attractiveness, by Application, 2018–2026
Figure 70: Latin America Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 71: Latin America Medical Device Contract Manufacturing Market Value Share (%), by Country/Sub-region, 2017 and 2026
Figure 72: Latin America Medical Device Contract Manufacturing Market Attractiveness, by Country/Sub-region, 2018–2026
Figure 73: Latin America Medical Device Contract Manufacturing Market Value Share (%), by Device Type, 2017 and 2026
Figure 74: Latin America Medical Device Contract Manufacturing Market Attractiveness, by Device Type, 2018–2026
Figure 75: Latin America Medical Device Contract Manufacturing Market Value Share (%), by Type of Manufacturing, 2017 and 2026
Figure 76: Latin America Medical Device Contract Manufacturing Market Attractiveness, by Type of Manufacturing, 2018–2026
Figure 77: Latin America Medical Device Contract Manufacturing Market Value Share (%), by Service, 2017 and 2026
Figure 78: Latin America Medical Device Contract Manufacturing Market Attractiveness, by Service, 2018–2026
Figure 79: Latin America Medical Device Contract Manufacturing Market Value Share (%), by Application, 2017 and 2026
Figure 80: Latin America Medical Device Contract Manufacturing Market Attractiveness, by Application, 2018–2026
Figure 81: Middle East & Africa Medical Device Contract Manufacturing Market Revenue (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2026
Figure 82: Middle East & Africa Medical Device Contract Manufacturing Market Value Share (%), by Country, 2017 and 2026
Figure 83: Middle East & Africa Medical Device Contract Manufacturing Market Attractiveness, by Country/Sub-region, 2018–2026
Figure 84: Middle East & Africa Medical Device Contract Manufacturing Market Value Share (%), by Type of Manufacturing, 2017 and 2026
Figure 85: Middle East & Africa Medical Device Contract Manufacturing Market Attractiveness, by Type of Manufacturing, 2018–2026
Figure 86: Middle East & Africa Medical Device Contract Manufacturing Market Value Share (%), by Service, 2017 and 2026
Figure 87: Middle East & Africa Medical Device Contract Manufacturing Market Attractiveness, by Service, 2018–2026
Figure 88: Middle East & Africa Medical Device Contract Manufacturing Market Value Share (%), by Application, 2017 and 2026
Figure 89: Middle East & Africa Medical Device Contract Manufacturing Market Attractiveness, by Application, 2018–2026
Figure 90: Middle East & Africa Medical Device Contract Manufacturing Market Value Share (%), by Device Type, 2017 and 2026
Figure 91: Middle East & Africa Medical Device Contract Manufacturing Market Attractiveness, by Device Type, 2018–2026
Figure 92: Global Medical Device Contract Manufacturing Market Analysis, by Top Company Ranking, 2017