Reports
Reports
Analysts’ Viewpoint
Rise in prevalence of HIV/AIDS is expected to propel the HIV/AIDS diagnostics market size during the forecast period. HIV/AIDS tests identify antibodies in blood or oral fluids. Most assays that are used to detect Nucleic Acids (NAT) are complex and technically demanding.
Increase in awareness about HIV/AIDS diagnosis is expected to augment market expansion in the near future. Over-the-counter HIV self-tests are gaining traction, as they help overcome the stigma associated with HIV testing. R&D of new diagnostic technologies is likely to offer lucrative growth opportunities for vendors in the industry. Key players are launching advanced rapid diagnostic tests in order to increase their HIV/AIDS diagnostics market share.
Human Immunodeficiency Virus (HIV) is a virus that attacks the immune system, specifically CD4 cells (T cells). Untreated HIV can lead to Acquired Immunodeficiency Syndrome (AIDS), a condition in which the immune system is severely damaged and incapable of fighting infections and diseases.
HIV is primarily transmitted through the exchange of bodily fluids such as blood, semen, vaginal secretions, and breast milk. Sexual contact, injection drug use, and mother-to-child transmission (during birth or breastfeeding) are the most common modes of transmission.
HIV can also be transmitted through the use of contaminated needles or equipment for tattooing, body piercing, or acupuncture. Organ transplantation or blood transfusion can also transmit HIV to a recipient. Stringent screening of blood and organ donors can limit this type of transmission.
The ongoing global HIV/AIDS epidemic and the need for accurate disease diagnosis and monitoring are estimated to boost the demand for HIV/AIDS diagnostics. According to the World Health Organization (WHO), approximately 38.8 million people worldwide suffered from HIV in 2021. The number of cases is increasing steadily, as the virus continues to spread. As per the Centers for Disease Control and Prevention (CDC), around 38,000 new HIV infections were reported in the U.S. in 2019.
According to the same source, HIV affects certain groups disproportionately in the U.S., including men who have had intercourse with men, people who inject drugs, and transgender women. In 2019, it was estimated that approximately 1.2 million people in the country were living with HIV, with men constituting the vast majority. Thus, high incidence of HIV/AIDS is anticipated to contribute to HIV/AIDS diagnostics market growth in the near future.
Development of new diagnostic technologies and surge in accessibility of testing in emerging economies are expected to offer lucrative growth opportunities for vendors in HIV/AIDS diagnostics industry. Key players are launching advanced products such as rapid tests and nucleic acid-based assay (Simple Amplification-based Assay (SAMBA) to broaden their customer base.
Implementation of POC diagnostic standards is also expected to augment the market size in the next few years. According to the WHO, an ideal POC diagnostic must be Affordable, Sensitive, Specific, User-friendly, Rapid/Robust, Equipment-free, and Deliverable (ASSURED). As per the WHO, in 2021, approximately 76% of people living with HIV globally were aware of their HIV-positive status. This represents an increase from previous years and demonstrates the success of efforts to raise awareness and testing for HIV/AIDS.
Implementation of HIV testing programs in several healthcare settings has led to a significant rise in awareness about the disease and testing of HIV/AIDS. These programs are designed to increase the number of people being tested for HIV and make testing more accessible.
The CDC recommends that everyone between the ages of 13 and 64 be tested for HIV at least once as part of routine health care. It also encourages more frequent testing for those at higher risk of HIV infection. According to the CDC, approximately 85% of people living with HIV in the U.S. received an HIV diagnosis in 2019.
According to the European Centre for Disease Prevention and Control (ECDC), in 2021, approximately 78% of HIV-positive people in the European Union and the European Economic Area were aware of their HIV status. This can be ascribed to the successful efforts of various governments in Europe to raise HIV/AIDS awareness and testing in the region. Thus, growth in awareness about HIV/AIDS is projected to boost the HIV/AIDS diagnostics market progress in the near future.
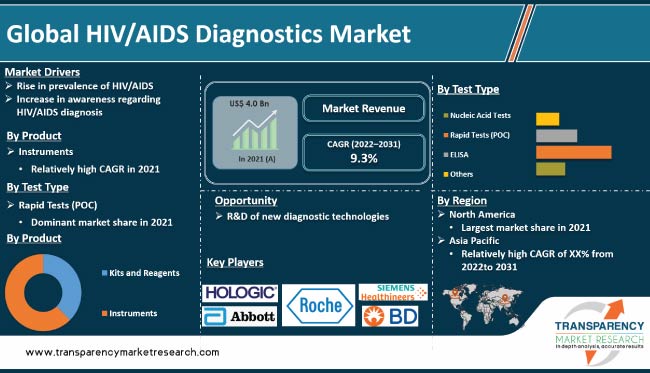
According to the latest HIV/AIDS diagnostics market trends, the instruments product segment held major share of the industry in 2021. Rise in number of HIV patients is expected to drive the demand for HIV diagnostic instruments.
HIV diagnostic testing employs a variety of instruments, including ELISA and Western Blot. Some instrument-based tests, such as Polymerase Chain Reaction (PCR), are extremely sensitive and can detect very low levels of HIV in a person's blood.
Vendors in the HIV/AIDS diagnostics business are launching new products in the instruments product segment. In August 2022, Molbio Diagnostics launched the Truenat RT-PCR Test for HIV diagnosis. Truenat is a portable, battery-powered, IoT-enabled, real-time PCR platform for Point-of-Care (POC) testing with a sample-to-result time of less than an hour.
Based on test type, the rapid tests (POC) segment is likely to dominate the industry during the forecast period, as per the latest HIV/AIDS diagnostics market analysis. Rapid HIV tests can be performed at POC, which is a more convenient option for patients than having to go to a separate laboratory. Furthermore, rapid tests are less expensive than traditional laboratory tests.
North America is expected to hold the largest share of the HIV/AIDS diagnostics industry during the forecast period. The region dominated the market with 30.0% share in 2021. Increase in incidence of HIV/AIDS, presence of key vendors, and rise in investment in R&D are boosting market statistics in the region.
The industry in Asia Pacific is projected to grow at the fastest rate during the forecast period due to high incidence of HIV/AIDS in the region.
The global industry is fragmented, with the presence of a large number of vendors. Most of the companies are increasing their market share through significant investment in R&D of new tests. Expansion of product portfolio and mergers & acquisitions are key strategies adopted by vendors.
Hologic, Inc. Abbott, Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche Ltd., OraSure Technologies, Inc., Siemens Healthcare GmbH, Chembio Diagnostics, Inc., Danaher Corporation, and Becton, Dickinson and Company are prominent market entities.
Each of these players has been profiled in the market report based on parameters such as company overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Market Size Value in 2021 |
US$ 4.0 Bn |
|
Market Forecast Value in 2031 |
More than US$ 9.6 Bn |
|
Growth Rate (CAGR) |
9.3% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2020 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Furthermore, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
It was valued at US$ 4.0 Bn in 2021.
It is projected to reach more than US$ 9.6 Bn by 2031.
The CAGR is anticipated to be 9.3% from 2022 to 2031.
Rise in prevalence of HIV/AIDS and increase in awareness about HIV/AIDS diagnosis.
North America is likely to account for major share from 2022 to 2031.
Hologic, Inc. Abbott, Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche Ltd., OraSure Technologies, Inc., Siemens Healthcare GmbH, Chembio Diagnostics, Inc., Danaher Corporation, and Becton, Dickinson and Company.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global HIV/AIDS Diagnostics Market
4. Market Overview
4.1. Introduction
4.1.1. Definition
4.1.2. Industry Evolution/Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global HIV/AIDS Diagnostics Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projection (US$ Mn)
4.4.2. Market Volume/Unit Shipments Projection
4.5. Porter’s Five Forces Analysis
5. Key Insights
5.1. HIV Disease Epidemiology
5.2. Technological Advancements
5.3. Key Mergers & Acquisitions
5.4. COVID-19 Pandemic Impact on Industry (Value Chain and Short/Mid/Long-term Impact)
6. Global HIV/AIDS Diagnostics Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Product, 2017–2031
6.3.1. Kits and Reagents
6.3.2. Instruments
6.4. Market Attractiveness Analysis, by Product
7. Global HIV/AIDS Diagnostics Market Analysis and Forecast, by Test Type
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Test Type, 2017–2031
7.3.1. Nucleic Acid Tests
7.3.2. Rapid Tests (POC)
7.3.3. ELISA
7.3.4. Others
7.4. Market Attractiveness Analysis, by Test Type
8. Global HIV/AIDS Diagnostics Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by End-user, 2017–2031
8.3.1. Hospitals
8.3.2. Diagnostic Laboratories
8.3.3. Academic and Research Institutes
8.3.4. Others
8.4. Market Attractiveness Analysis, by End-user
9. Global HIV/AIDS Diagnostics Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region, 2017–2031
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America HIV/AIDS Diagnostics Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Product, 2017–2031
10.2.1. Kits and Reagents
10.2.2. Instruments
10.3. Market Value Forecast, by Test Type, 2017–2031
10.3.1. Nucleic Acid Tests
10.3.2. Rapid Tests (POC)
10.3.3. ELISA
10.3.4. Others
10.4. Market Value Forecast, by End-user, 2017–2031
10.4.1. Hospitals
10.4.2. Diagnostic Laboratories
10.4.3. Academic and Research Institutes
10.4.4. Others
10.5. Market Value Forecast, by Country, 2017–2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Product
10.6.2. By Test Type
10.6.3. By End-user
10.6.4. By Country
11. Europe HIV/AIDS Diagnostics Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Product, 2017–2031
11.2.1. Kits and Reagents
11.2.2. Instruments
11.3. Market Value Forecast, by Test Type, 2017–2031
11.3.1. Nucleic Acid Tests
11.3.2. Rapid Tests (POC)
11.3.3. ELISA
11.3.4. Others
11.4. Market Value Forecast, by End-user, 2017–2031
11.4.1. Hospitals
11.4.2. Diagnostic Laboratories
11.4.3. Academic and Research Institutes
11.4.4. Others
11.5. Market Value Forecast, by Country/Sub-region, 2017–2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Italy
11.5.5. Spain
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Product
11.6.2. By Test Type
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific HIV/AIDS Diagnostics Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Product, 2017–2031
12.2.1. Kits and Reagents
12.2.2. Instruments
12.3. Market Value Forecast, by Test Type, 2017–2031
12.3.1. Nucleic Acid Tests
12.3.2. Rapid Tests (POC)
12.3.3. ELISA
12.3.4. Others
12.4. Market Value Forecast, by End-user, 2017–2031
12.4.1. Hospitals
12.4.2. Diagnostic Laboratories
12.4.3. Academic and Research Institutes
12.4.4. Others
12.5. Market Value Forecast, by Country/Sub-region, 2017–2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Product
12.6.2. By Test Type
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America HIV/AIDS Diagnostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Product, 2017–2031
13.2.1. Kits and Reagents
13.2.2. Instruments
13.3. Market Value Forecast, by Test Type, 2017–2031
13.3.1. Nucleic Acid Tests
13.3.2. Rapid Tests (POC)
13.3.3. ELISA
13.3.4. Others
13.4. Market Value Forecast, by End-user, 2017–2031
13.4.1. Hospitals
13.4.2. Diagnostic Laboratories
13.4.3. Academic and Research Institutes
13.4.4. Others
13.5. Market Value Forecast, by Country/Sub-region, 2017–2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Product
13.6.2. By Test Type
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa HIV/AIDS Diagnostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Product, 2017–2031
14.2.1. Kits and Reagents
14.2.2. Instruments
14.3. Market Value Forecast, by Test Type, 2017–2031
14.3.1. Nucleic Acid Tests
14.3.2. Rapid Tests (POC)
14.3.3. ELISA
14.3.4. Others
14.4. Market Value Forecast, by End-user, 2017–2031
14.4.1. Hospitals
14.4.2. Diagnostic Laboratories
14.4.3. Academic and Research Institutes
14.4.4. Others
14.5. Market Value Forecast, by Country/Sub-region, 2017–2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Product
14.6.2. By Test Type
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player – Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company (2021)
15.3. Company Profiles
15.3.1. Hologic, Inc
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Financial Overview
15.3.1.4. SWOT Analysis
15.3.1.5. Strategic Overview
15.3.2. Abbott
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. SWOT Analysis
15.3.2.5. Strategic Overview
15.3.3. Bio-Rad Laboratories, Inc.
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Financial Overview
15.3.3.4. SWOT Analysis
15.3.3.5. Strategic Overview
15.3.4. F. Hoffmann-La Roche Ltd.
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. SWOT Analysis
15.3.4.5. Strategic Overview
15.3.5. OraSure Technologies, Inc.
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. SWOT Analysis
15.3.6. Siemens Healthcare GmbH
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Financial Overview
15.3.6.4. SWOT Analysis
15.3.7. Chembio Diagnostics, Inc.
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. SWOT Analysis
15.3.8. Danaher Corporation
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Financial Overview
15.3.8.4. SWOT Analysis
15.3.9. Becton, Dickinson and Company
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. Financial Overview
15.3.9.4. SWOT Analysis
List of Tables
Table 01: Global HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 02: Global HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017–2031
Table 03: Global HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 04: Global HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 05: North America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 06: North America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 07: North America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017–2031
Table 08: North America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 09: Europe HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 10: Europe HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 11: Europe HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017–2031
Table 12: Europe HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 13: Asia Pacific HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 14: Asia Pacific HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 15: Asia Pacific HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017–2031
Table 16: Asia Pacific HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 17: Latin America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 18: Latin America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 19: Latin America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017–2031
Table 20: Latin America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 21: Middle East & Africa HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 22: Middle East & Africa HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 23: Middle East & Africa HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017–2031
Table 24: Middle East & Africa HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
List of Figures
Figure 01: Market Share, By Product
Figure 02: Market Share, By Test Type
Figure 03: Market Share, By End-user
Figure 04: Market Share, By Region
Figure 05: Figure 05: Global HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 06: Figure 06: Global HIV/AIDS Diagnostics Market Value Share, by Product (2021)
Figure 07: Figure 07: Global HIV/AIDS Diagnostics Market Value Share, by End-user (2021)
Figure 08: Global HIV/AIDS Diagnostics Market Value Share, by Test Type (2021)
Figure 09: Global HIV/AIDS Diagnostics Market Value Share, by Region (2021)
Figure 10: Global HIV/AIDS Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 11: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Kits and Reagents, 2017–2031
Figure 12: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Instruments, 2017–2031
Figure 13: Global HIV/AIDS Diagnostics Market Attractiveness, by Product, 2022–2031
Figure 14: Global HIV/AIDS Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 15: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Nucleic Acid Tests, 2017–2031
Figure 16: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Rapid Tests (POC), 2017–2031
Figure 17: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by ELISA, 2017–2031
Figure 18: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Others, 2017–2031
Figure 19: Global HIV/AIDS Diagnostics Market Attractiveness, by Test Type, 2022–2031
Figure 20: Global HIV/AIDS Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 21: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Hospitals, 2017–2031
Figure 22: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Diagnostic Laboratories, 2017–2031
Figure 23: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Academic and Research Institutes, 2017–2031
Figure 24: Global HIV/AIDS Diagnostics Market Revenue (US$ Mn), by Others, 2017–2031
Figure 25: Global HIV/AIDS Diagnostics Market Attractiveness, by End-user, 2022–2031
Figure 26: Global HIV/AIDS Diagnostics Market Value Share Analysis, by Region, 2021 and 2031
Figure 27: Global HIV/AIDS Diagnostics Market Attractiveness, by Region, 2022-2031
Figure 28: North America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 29: North America HIV/AIDS Diagnostics Market Value Share (%), by Country, 2021 and 2031
Figure 30: North America HIV/AIDS Diagnostics Market Attractiveness, by Country, 2022–2031
Figure 31: North America HIV/AIDS Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 32: North America HIV/AIDS Diagnostics Market Attractiveness, by Product, 2022–2031
Figure 33: North America HIV/AIDS Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 34: North America HIV/AIDS Diagnostics Market Attractiveness, by Test Type, 2022–2031
Figure 35: North America HIV/AIDS Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 36: North America HIV/AIDS Diagnostics Market Attractiveness, by End-user, 2022–2031
Figure 37: Europe HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 38: Europe HIV/AIDS Diagnostics Market Value Share (%), by Country/Sub-region, 2021 and 2031
Figure 39: Europe HIV/AIDS Diagnostics Market Attractiveness, by Country/Sub-region, 2022–2031
Figure 40: Europe HIV/AIDS Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 41: Europe HIV/AIDS Diagnostics Market Attractiveness, by Product, 2022–2031
Figure 42: Europe HIV/AIDS Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 43: Europe HIV/AIDS Diagnostics Market Attractiveness, by Test Type, 2022–2031
Figure 44: Europe HIV/AIDS Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 45: Europe HIV/AIDS Diagnostics Market Attractiveness, by End-user, 2022–2031
Figure 46: Asia Pacific HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 47: Asia Pacific HIV/AIDS Diagnostics Market Value Share (%), by Country/Sub-region, 2021 and 2031
Figure 48: Asia Pacific HIV/AIDS Diagnostics Market Attractiveness, by Country/Sub-region, 2022–2031
Figure 49: Asia Pacific HIV/AIDS Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 50: Asia Pacific HIV/AIDS Diagnostics Market Attractiveness, by Product, 2022–2031
Figure 51: Asia Pacific HIV/AIDS Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 52: Asia Pacific HIV/AIDS Diagnostics Market Attractiveness, by Test Type, 2022–2031
Figure 53: Asia Pacific HIV/AIDS Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 54: Asia Pacific HIV/AIDS Diagnostics Market Attractiveness, by End-user, 2022–2031
Figure 55: Latin America HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 56: Latin America HIV/AIDS Diagnostics Market Value Share (%), by Country/Sub-region, 2021 and 2031
Figure 57: Latin America HIV/AIDS Diagnostics Market Attractiveness, by Country/Sub-region, 2022–2031
Figure 58: Latin America HIV/AIDS Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 59: Latin America HIV/AIDS Diagnostics Market Attractiveness, by Product, 2022–2031
Figure 60: Latin America HIV/AIDS Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 61: Latin America HIV/AIDS Diagnostics Market Attractiveness, by Test Type, 2022–2031
Figure 62: Latin America HIV/AIDS Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 63: Latin America HIV/AIDS Diagnostics Market Attractiveness, by End-user, 2022–2031
Figure 64: Middle East & Africa HIV/AIDS Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 65: Middle East & Africa HIV/AIDS Diagnostics Market Value Share (%), by Country/Sub-region, 2021 and 2031
Figure 66: Middle East & Africa HIV/AIDS Diagnostics Market Attractiveness, by Country/Sub-region, 2022–2031
Figure 67: Middle East & Africa HIV/AIDS Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 68: Middle East & Africa HIV/AIDS Diagnostics Market Attractiveness, by Product, 2022–2031
Figure 69: Middle East & Africa HIV/AIDS Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 70: Middle East & Africa HIV/AIDS Diagnostics Market Attractiveness, by Test Type, 2022–2031
Figure 71: Middle East & Africa HIV/AIDS Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 72: Middle East & Africa HIV/AIDS Diagnostics Market Attractiveness, by End-user, 2022–2031
Figure 73: Global HIV/AIDS Diagnostics Market Share Analysis, by Company, 2021