Reports
Reports
Increasing stringent government regulations and demand for local representation are major factors driving regulatory affairs outsourcing market demand. As companies strive to accelerate product approvals and navigate complex, region-specific regulations, outsourcing regulatory functions has become a strategic necessity. Additionally, the demand for localized expertise and on-the-ground presence in emerging markets such as Asia Pacific, Latin America, and the Middle East is a key factor propelling market expansion.
.jpg)
In line with the latest regulatory affairs outsourcing market trends, leading firms are broadening their service offerings to include end-to-end regulatory consulting, clinical trial support, pharmacovigilance, and compliance management to cater to diverse client needs and become one-stop solutions.
Regulatory affairs outsourcing involves partnering with specialized third-party service providers to manage and navigate the complex regulatory requirements that govern the development, approval, and marketing of pharmaceutical, biotechnology, and medical device products. This strategy enables companies to leverage external expertise in regulatory submissions, compliance monitoring, and documentation management, ensuring adherence to global regulatory standards.
Outsourcing regulatory affairs helps organizations reduce operational costs, accelerate product approvals, and mitigate risks associated with regulatory non-compliance. It is especially beneficial for small to mid-sized companies lacking in-house regulatory resources or for large firms aiming to optimize efficiency. With increasing regulatory complexity and evolving guidelines worldwide, regulatory affairs outsourcing is becoming a critical component in the successful commercialization of healthcare products.
| Attribute | Detail |
|---|---|
| Regulatory Affairs Outsourcing Market Drivers |
|
As new tech, medicines, and medical gadgets keep popping up quickly, the rules around them are getting more complicated and stricter. For companies in pharma and biotech, it’s really hard to keep up with all these changing rules and make sure they’re following everything at every step - from developing to selling their products. The approval process is taking longer now because of more data needed. Missing a step or making a mistake can cause delays in launching new products and slow down business. Many companies choose to outsource some of their regulatory work.
This way, they can work with experts who keep up with the latest rules and regulations all the time. These specialists understand the changing guidelines through ongoing research and talking to stakeholders. They can help guide companies ahead of time and make sure all submissions are up-to-date, speeding up approval times. By outsourcing the routine regulatory tasks, companies can free up their staff to focus on more strategic priorities and grow their business more effectively.
For instance, in November 2024, the Medical Device Coordination Group (MDCG) issued Q&A addressing practical aspects of the gradual roll-out of Eudamed under Regulations (EU) 2017/745 (MDR) and (EU) 2017/746 (IVDR), as amended by Regulation (EU) 2024/1860. The Q&A specify that Eudamed includes six modules and clarify that the obligations and requirements for each module will become applicable six months after the publication of the notice confirming the functionality of the module in the Official Journal.
As new markets emerge globally, companies are increasingly seeking international expansion. However, regulatory rules and approval processes vary significantly across countries due to differences in healthcare systems, policies, and cultural factors. To comply with each country’s requirements, companies need a thorough understanding of local regulations along with on-site support, including facilities and staff. This poses a considerable challenge for multinational companies managing global operations. Regulatory outsourcing addresses this by providing access to experts well-versed in local laws. These outsourcing partners establish local offices and hire native representatives who can liaise directly with local authorities on their behalf.
Moreover, the expansion of outsourcing services by key market players is expected to drive growth in the regulatory affairs outsourcing industry sector. For example, in March 2025, ICON PLC expanded its outsourcing services within the Asia Pacific region. This strategic move responds directly to the growing demand for regulatory consultation and clinical trial support in rapidly developing markets such as China and India.
Product registration and clinical trial applications segment is expected to lead service type market segment of the regulatory affairs outsourcing industry. The growth is attributed owing to the increasing number of clinical trials across the globe, stringent regulations in developed markets, and legal/regulatory reforms in emerging markets such as Asia Pacific are driving the outsourcing trend for clinical trial applications. The segment growth is owing to increasing demand for legal representatives worldwide on account of the globalization of medical devices and pharmaceutical companies.
The regulations are very complex and ever-changing. The changing regulatory landscape in regions such as Asia Pacific, MEA, and Latin America increases the demand for local experts for legal representation for obtaining regulatory approvals and custom clearance. These factors are promoting the demand for legal representation services across the world.
| Attribute | Detail |
|---|---|
| Leading Region | North America |
According to the latest regulatory affairs outsourcing market analysis North America region is expected to dominate the market share with an estimated market share of 39.4% in 2024. Presence of established pharmaceutical companies, stringent regulations such as FDA and presence of leading contract research organizations (CROs) offering end-to-end regulatory solutions are some of the major factors driving market growth. Furthermore, high concentration of clinical trials in this region increases the need for regulatory expertise. Additionally, Asia Pacific region is also poised to be the fastest growing market during the forecast period.
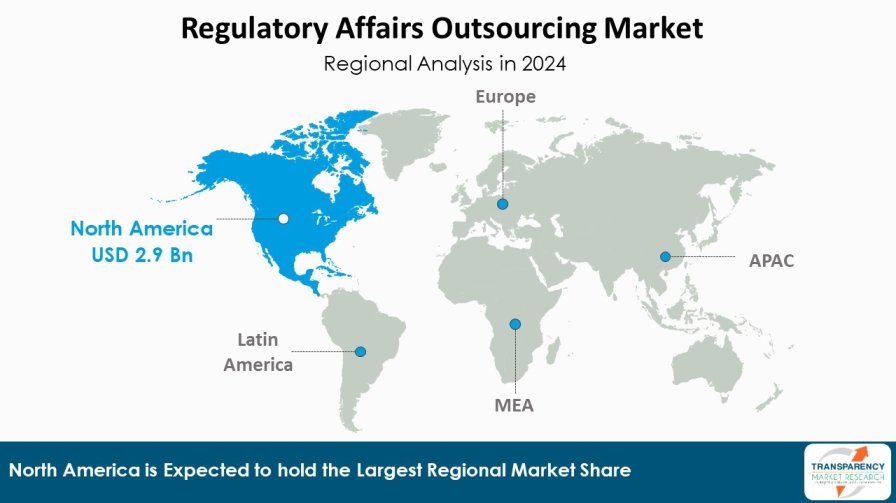
Countries like China, India and Japan are emerging as global sourcing destinations for regulatory affairs due to increasing investments in healthcare infrastructure and pharmaceutical manufacturing capabilities. In addition, Asia Pacific companies are actively enhancing their global regulatory networks to cater growing international marketing authorizations for their products. While pricing of services is relatively lower compared to mature markets, the region offers high future growth potential backed by improving quality standards.
Leading companies are partnering with hospitals, specialty clinics, and research institutes to expand inorganically. Accell Clinical Research, LLC, Genpact, CRITERIUM, INC, Promedica International, WuXi AppTec, Medpace, Charles River Laboratories, ICON plc, Labcorp Drug Development, Parexel International Corporation, Freyr, PHARMALEX GMBH, and other prominent players are the prominent regulatory affairs outsourcing market players.
Each of these players has been have been profiled in the regulatory affairs outsourcing market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
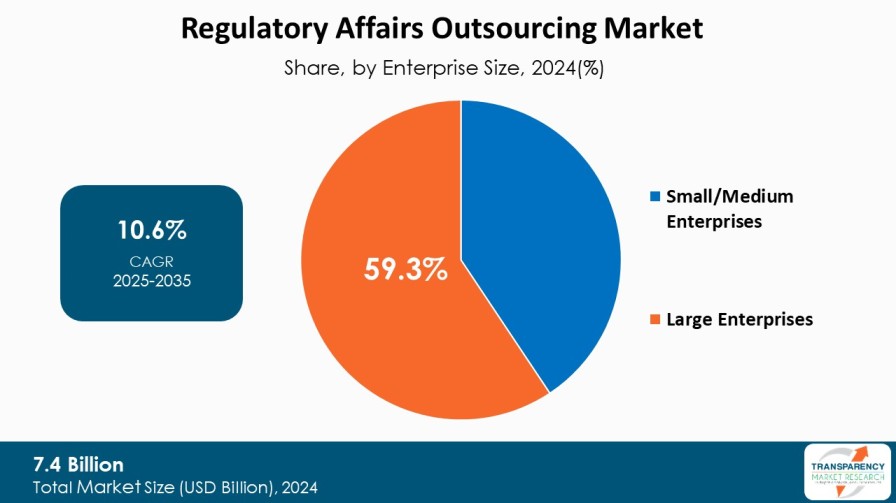
| Size in 2024 | US$ 7.4 Bn |
| Forecast Value in 2035 | More than US$ 22.3 Bn |
| CAGR | 10.6% |
| Forecast Period | 2025-2035 |
| Historical Data Available for | 2020-2023 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
The global regulatory affairs outsourcing market was valued at US$ 7.4 Bn in 2024.
Regulatory affairs outsourcing business is projected to cross US$ 22.3 Bn by the end of 2035.
Increasing stringent government regulations and demand for local representation to expand into new high potential markets worldwide.
The CAGR is anticipated to be 10.6% from 2025 to 2035.
North America is expected to account for the largest share from 2025 to 2035.
Accell Clinical Research, LLC, Genpact, CRITERIUM, INC, Promedica International, WuXi AppTec, Medpace, Charles River Laboratories, ICON plc, Labcorp Drug Development, Parexel International Corporation, Freyr, and PHARMALEX GMBH are the prominent Regulatory Affairs Outsourcing market players.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary : Global Regulatory Affairs Outsourcing Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, 2020 to 2035
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Overview on Current Trends in Regulatory Affairs Outsourcing Market Models
5.2. PORTER’s Five Forces Analysis
5.3. PESTEL Analysis
5.4. Regulatory Scenario across Key Regions/Country
5.5. Service Launches/Approvals by the Major Players/Regulatory Authority
5.6. Mergers and Acquisition Scenario for Regulatory Affairs Outsourcing Market
5.7. Benchmarking of the Services Offered by the Competitors
5.8. Investment Trends in Regulatory Affairs Outsourcing Services
5.9. Go-to-Market Strategy for New Market Entrants
6. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, By Service Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast By Service Type, 2020 to 2035
6.3.1. Regulatory Consulting and Legal Representation
6.3.2. Product Registration and Clinical Trial Applications
6.3.3. Regulatory Writing and Publishing
6.3.4. Regulatory Submission
6.3.5. Regulatory Operations
6.3.6. Others
6.4. Market Attractiveness By Service Type
7. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, By Product Development Stage
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast By Product Development Stage, 2020 to 2035
7.3.1. Preclinical
7.3.2. Clinical
7.3.3. PMA (Post Market Authorization)
7.4. Market Attractiveness By Product Development Stage
8. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, By Enterprise Size
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast By Enterprise Size, 2020 to 2035
8.3.1. Small/Medium Enterprises
8.3.2. Large Enterprises
8.4. Market Attractiveness By Enterprise Size
9. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, By Therapeutic Area
9.1. Introduction & Definition
9.2. Key Findings / Developments
9.3. Market Value Forecast By Therapeutic Area, 2020 to 2035
9.3.1. Oncology
9.3.2. Neurology
9.3.3. Cardiology
9.3.4. Immunology
9.3.5. Dermatology
9.3.6. Others
9.4. Market Attractiveness By Therapeutic Area
10. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, By End-user
10.1. Introduction & Definition
10.2. Key Findings / Developments
10.3. Market Value Forecast By End-user, 2020 to 2035
10.3.1. Medical Device Companies
10.3.2. Biopharma & Pharmaceutical Companies
10.3.3. Others
10.4. Market Attractiveness By End-user
11. Global Regulatory Affairs Outsourcing Market Analysis and Forecasts, By Region
11.1. Key Findings
11.2. Market Value Forecast By Region
11.2.1. North America
11.2.2. Europe
11.2.3. Asia Pacific
11.2.4. Latin America
11.2.5. Middle East & Africa
11.3. Market Attractiveness By Region
12. North America Regulatory Affairs Outsourcing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast By Service Type, 2020 to 2035
12.2.1. Regulatory Consulting and Legal Representation
12.2.2. Product Registration and Clinical Trial Applications
12.2.3. Regulatory Writing and Publishing
12.2.4. Regulatory Submission
12.2.5. Regulatory Operations
12.2.6. Others
12.3. Market Value Forecast By Product Development Stage, 2020 to 2035
12.3.1. Preclinical
12.3.2. Clinical
12.3.3. PMA (Post Market Authorization)
12.4. Market Value Forecast By Enterprise Size, 2020 to 2035
12.4.1. Small/Medium Enterprises
12.4.2. Large Enterprises
12.5. Market Value Forecast By Therapeutic Area, 2020 to 2035
12.5.1. Oncology
12.5.2. Neurology
12.5.3. Cardiology
12.5.4. Immunology
12.5.5. Dermatology
12.5.6. Others
12.6. Market Value Forecast By End-user, 2020 to 2035
12.6.1. Medical Device Companies
12.6.2. Biopharma & Pharmaceutical Companies
12.6.3. Others
12.7. Market Value Forecast By Country, 2020 to 2035
12.7.1. U.S.
12.7.2. Canada
12.8. Market Attractiveness Analysis
12.8.1. By Service Type
12.8.2. By Product Development Stage
12.8.3. By Enterprise Size
12.8.4. By Therapeutic Area
12.8.5. By End-user
12.8.6. By Country
13. Europe Regulatory Affairs Outsourcing Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast By Service Type, 2020 to 2035
13.2.1. Regulatory Consulting and Legal Representation
13.2.2. Product Registration and Clinical Trial Applications
13.2.3. Regulatory Writing and Publishing
13.2.4. Regulatory Submission
13.2.5. Regulatory Operations
13.2.6. Others
13.3. Market Value Forecast By Product Development Stage, 2020 to 2035
13.3.1. Preclinical
13.3.2. Clinical
13.3.3. PMA (Post Market Authorization)
13.4. Market Value Forecast By Enterprise Size, 2020 to 2035
13.4.1. Small/Medium Enterprises
13.4.2. Large Enterprises
13.5. Market Value Forecast By Therapeutic Area, 2020 to 2035
13.5.1. Oncology
13.5.2. Neurology
13.5.3. Cardiology
13.5.4. Immunology
13.5.5. Dermatology
13.5.6. Others
13.6. Market Value Forecast By End-user, 2020 to 2035
13.6.1. Medical Device Companies
13.6.2. Biopharma & Pharmaceutical Companies
13.6.3. Others
13.7. Market Value Forecast By Country/Sub-region, 2020 to 2035
13.7.1. Germany
13.7.2. UK
13.7.3. France
13.7.4. Italy
13.7.5. Spain
13.7.6. Switzerland
13.7.7. The Netherlands
13.7.8. Rest of Europe
13.8. Market Attractiveness Analysis
13.8.1. By Service Type
13.8.2. By Product Development Stage
13.8.3. By Enterprise Size
13.8.4. By Therapeutic Area
13.8.5. By End-user
13.8.6. By Country/Sub-region
14. Asia Pacific Regulatory Affairs Outsourcing Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast By Service Type, 2020 to 2035
14.2.1. Regulatory Consulting and Legal Representation
14.2.2. Product Registration and Clinical Trial Applications
14.2.3. Regulatory Writing and Publishing
14.2.4. Regulatory Submission
14.2.5. Regulatory Operations
14.2.6. Others
14.3. Market Value Forecast By Product Development Stage, 2020 to 2035
14.3.1. Preclinical
14.3.2. Clinical
14.3.3. PMA (Post Market Authorization)
14.4. Market Value Forecast By Enterprise Size, 2020 to 2035
14.4.1. Small/Medium Enterprises
14.4.2. Large Enterprises
14.5. Market Value Forecast By Therapeutic Area, 2020 to 2035
14.5.1. Oncology
14.5.2. Neurology
14.5.3. Cardiology
14.5.4. Immunology
14.5.5. Dermatology
14.5.6. Others
14.6. Market Value Forecast By End-user, 2020 to 2035
14.6.1. Medical Device Companies
14.6.2. Biopharma & Pharmaceutical Companies
14.6.3. Others
14.7. Market Value Forecast By Country/Sub-region, 2020 to 2035
14.7.1. China
14.7.2. Japan
14.7.3. India
14.7.4. Australia & New Zealand
14.7.5. South Korea
14.7.6. Rest of Asia Pacific
14.8. Market Attractiveness Analysis
14.8.1. By Service Type
14.8.2. By Product Development Stage
14.8.3. By Enterprise Size
14.8.4. By Therapeutic Area
14.8.5. By End-user
14.8.6. By Country/Sub-region
15. Latin America Regulatory Affairs Outsourcing Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast By Service Type, 2020 to 2035
15.2.1. Regulatory Consulting and Legal Representation
15.2.2. Product Registration and Clinical Trial Applications
15.2.3. Regulatory Writing and Publishing
15.2.4. Regulatory Submission
15.2.5. Regulatory Operations
15.2.6. Others
15.3. Market Value Forecast By Product Development Stage, 2020 to 2035
15.3.1. Preclinical
15.3.2. Clinical
15.3.3. PMA (Post Market Authorization)
15.4. Market Value Forecast By Enterprise Size, 2020 to 2035
15.4.1. Small/Medium Enterprises
15.4.2. Large Enterprises
15.5. Market Value Forecast By Therapeutic Area, 2020 to 2035
15.5.1. Oncology
15.5.2. Neurology
15.5.3. Cardiology
15.5.4. Immunology
15.5.5. Dermatology
15.5.6. Others
15.6. Market Value Forecast By End-user, 2020 to 2035
15.6.1. Medical Device Companies
15.6.2. Biopharma & Pharmaceutical Companies
15.6.3. Others
15.7. Market Value Forecast By Country/Sub-region, 2020 to 2035
15.7.1. Brazil
15.7.2. Mexico
15.7.3. Argentina
15.7.4. Rest of Latin America
15.8. Market Attractiveness Analysis
15.8.1. By Service Type
15.8.2. By Product Development Stage
15.8.3. By Enterprise Size
15.8.4. By Therapeutic Area
15.8.5. By End-user
15.8.6. By Country/Sub-region
16. Middle East & Africa Regulatory Affairs Outsourcing Market Analysis and Forecast
16.1. Introduction
16.1.1. Key Findings
16.2. Market Value Forecast By Service Type, 2020 to 2035
16.2.1. Regulatory Consulting and Legal Representation
16.2.2. Product Registration and Clinical Trial Applications
16.2.3. Regulatory Writing and Publishing
16.2.4. Regulatory Submission
16.2.5. Regulatory Operations
16.2.6. Others
16.3. Market Value Forecast By Product Development Stage, 2020 to 2035
16.3.1. Preclinical
16.3.2. Clinical
16.3.3. PMA (Post Market Authorization)
16.4. Market Value Forecast By Enterprise Size, 2020 to 2035
16.4.1. Small/Medium Enterprises
16.4.2. Large Enterprises
16.5. Market Value Forecast By Therapeutic Area, 2020 to 2035
16.5.1. Oncology
16.5.2. Neurology
16.5.3. Cardiology
16.5.4. Immunology
16.5.5. Dermatology
16.5.6. Others
16.6. Market Value Forecast By End-user, 2020 to 2035
16.6.1. Medical Device Companies
16.6.2. Biopharma & Pharmaceutical Companies
16.6.3. Others
16.7. Market Value Forecast By Country/Sub-region, 2020 to 2035
16.7.1. GCC Countries
16.7.2. South Africa
16.7.3. Rest of Middle East & Africa
16.8. Market Attractiveness Analysis
16.8.1. By Service Type
16.8.2. By Product Development Stage
16.8.3. By Enterprise Size
16.8.4. By Therapeutic Area
16.8.5. By End-user
16.8.6. By Country/Sub-region
17. Competition Landscape
17.1. Market Player – Competition Matrix (By Tier and Size of companies)
17.2. Market Share Analysis By Company (2024)
17.3. Company Profiles
17.3.1. Accell Clinical Research, LLC
17.3.1.1. Company Overview
17.3.1.2. Financial Overview
17.3.1.3. Financial Overview
17.3.1.4. Business Strategies
17.3.1.5. Recent Developments
17.3.2. Genpact
17.3.2.1. Company Overview
17.3.2.2. Financial Overview
17.3.2.3. Financial Overview
17.3.2.4. Business Strategies
17.3.2.5. Recent Developments
17.3.3. CRITERIUM, INC
17.3.3.1. Company Overview
17.3.3.2. Financial Overview
17.3.3.3. Financial Overview
17.3.3.4. Business Strategies
17.3.3.5. Recent Developments
17.3.4. Promedica International
17.3.4.1. Company Overview
17.3.4.2. Financial Overview
17.3.4.3. Financial Overview
17.3.4.4. Business Strategies
17.3.4.5. Recent Developments
17.3.5. WuXi AppTec
17.3.5.1. Company Overview
17.3.5.2. Financial Overview
17.3.5.3. Financial Overview
17.3.5.4. Business Strategies
17.3.5.5. Recent Developments
17.3.6. Medpace
17.3.6.1. Company Overview
17.3.6.2. Financial Overview
17.3.6.3. Financial Overview
17.3.6.4. Business Strategies
17.3.6.5. Recent Developments
17.3.7. Charles River Laboratories
17.3.7.1. Company Overview
17.3.7.2. Financial Overview
17.3.7.3. Financial Overview
17.3.7.4. Business Strategies
17.3.7.5. Recent Developments
17.3.8. ICON plc
17.3.8.1. Company Overview
17.3.8.2. Financial Overview
17.3.8.3. Financial Overview
17.3.8.4. Business Strategies
17.3.8.5. Recent Developments
17.3.9. Parexel International Corporation
17.3.9.1. Company Overview
17.3.9.2. Financial Overview
17.3.9.3. Financial Overview
17.3.9.4. Business Strategies
17.3.9.5. Recent Developments
17.3.10. Freyr
17.3.10.1. Company Overview
17.3.10.2. Financial Overview
17.3.10.3. Financial Overview
17.3.10.4. Business Strategies
17.3.10.5. Recent Developments
17.3.11. PHARMALEX GMBH
17.3.11.1. Company Overview
17.3.11.2. Financial Overview
17.3.11.3. Financial Overview
17.3.11.4. Business Strategies
17.3.11.5. Recent Developments
17.3.12. Labcorp Drug Development
17.3.12.1. Company Overview
17.3.12.2. Financial Overview
17.3.12.3. Financial Overview
17.3.12.4. Business Strategies
17.3.12.5. Recent Developments
17.3.13. Other Prominent Players
17.3.13.1. Company Overview
17.3.13.2. Financial Overview
17.3.13.3. Financial Overview
17.3.13.4. Business Strategies
17.3.13.5. Recent Developments
List of Tables
Table 01: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Service Type, 2020 to 2035
Table 02: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Product Development Stage, 2020 to 2035
Table 03: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Enterprise Size, 2020 to 2035
Table 04: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Therapeutic Area, 2020 to 2035
Table 05: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 06: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Region, 2020 to 2035
Table 07: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Country, 2020 to 2035
Table 08: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Service Type, 2020 to 2035
Table 09: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Product Development Stage, 2020 to 2035
Table 10: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Enterprise Size, 2020 to 2035
Table 11: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Therapeutic Area, 2020 to 2035
Table 12: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 13: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 14: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Service Type, 2020 to 2035
Table 15: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Product Development Stage, 2020 to 2035
Table 16: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Enterprise Size, 2020 to 2035
Table 17: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Therapeutic Area, 2020 to 2035
Table 18: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 19: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 20: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Service Type, 2020 to 2035
Table 21: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Product Development Stage, 2020 to 2035
Table 22: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Enterprise Size, 2020 to 2035
Table 23: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Therapeutic Area, 2020 to 2035
Table 24: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 25: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 26: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Service Type, 2020 to 2035
Table 27: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Product Development Stage, 2020 to 2035
Table 28: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Enterprise Size, 2020 to 2035
Table 29: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Therapeutic Area, 2020 to 2035
Table 30: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
Table 31: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Country/Sub-region, 2020 to 2035
Table 32: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, by Service Type, 2020 to 2035
Table 33: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Product Development Stage, 2020 to 2035
Table 34: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Enterprise Size, 2020 to 2035
Table 35: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By Therapeutic Area, 2020 to 2035
Table 36: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, By End-user, 2020 to 2035
List of Figures
Figure 01: Global Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 02: Global Regulatory Affairs Outsourcing Market Value Share Analysis, By Service Type, 2024 and 2035
Figure 03: Global Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Service Type, 2025 to 2035
Figure 04: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Regulatory Consulting and Legal Representation, 2020 to 2035
Figure 05: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Product Registration and Clinical Trial Product Development Stages, 2020 to 2035
Figure 06: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Regulatory Writing and Publishing, 2020 to 2035
Figure 07: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Regulatory Submission, 2020 to 2035
Figure 08: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Regulatory Operations, 2020 to 2035
Figure 09: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Others, 2020 to 2035
Figure 10: Global Regulatory Affairs Outsourcing Market Value Share Analysis, By Product Development Stage, 2024 and 2035
Figure 11: Global Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Product Development Stage, 2025 to 2035
Figure 12: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Preclinical, 2020 to 2035
Figure 13: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Clinical, 2020 to 2035
Figure 14: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by PMA (Post Market Authorization), 2020 to 2035
Figure 15: Global Regulatory Affairs Outsourcing Market Value Share Analysis, By Enterprise Size, 2024 and 2035
Figure 16: Global Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Enterprise Size, 2025 to 2035
Figure 17: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Small/Medium Enterprises, 2020 to 2035
Figure 18: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Large Enterprises, 2020 to 2035
Figure 19: Global Regulatory Affairs Outsourcing Market Value Share Analysis, By Therapeutic Area, 2024 and 2035
Figure 20: Global Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Therapeutic Area, 2025 to 2035
Figure 21: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Oncology, 2020 to 2035
Figure 22: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Neurology, 2020 to 2035
Figure 23: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Cardiology, 2020 to 2035
Figure 24: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Immunology, 2020 to 2035
Figure 25: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Dermatology, 2020 to 2035
Figure 26: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Others, 2020 to 2035
Figure 27: Global Regulatory Affairs Outsourcing Market Value Share Analysis, By End-user, 2024 and 2035
Figure 28: Global Regulatory Affairs Outsourcing Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 29: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Medical Device Companies, 2020 to 2035
Figure 30: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Biopharma & Pharmaceutical Companies, 2020 to 2035
Figure 31: Global Regulatory Affairs Outsourcing Market Revenue (US$ Bn), by Others, 2020 to 2035
Figure 32: Global Regulatory Affairs Outsourcing Market Value Share Analysis, By Region, 2024 and 2035
Figure 33: Global Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Region, 2025 to 2035
Figure 34: North America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 35: North America Regulatory Affairs Outsourcing Market Value Share Analysis, by Country, 2024 and 2035
Figure 36: North America Regulatory Affairs Outsourcing Market Attractiveness Analysis, by Country, 2025 to 2035
Figure 37: North America Regulatory Affairs Outsourcing Market Value Share Analysis, By Service Type, 2024 and 2035
Figure 38: North America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Service Type, 2025 to 2035
Figure 39: North America Regulatory Affairs Outsourcing Market Value Share Analysis, By Product Development Stage, 2024 and 2035
Figure 40: North America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Product Development Stage, 2025 to 2035
Figure 41: North America Regulatory Affairs Outsourcing Market Value Share Analysis, By Enterprise Size, 2024 and 2035
Figure 42: North America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Enterprise Size, 2025 to 2035
Figure 43: North America Regulatory Affairs Outsourcing Market Value Share Analysis, By Therapeutic Area, 2024 and 2035
Figure 44: North America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Therapeutic Area, 2025 to 2035
Figure 45: North America Regulatory Affairs Outsourcing Market Value Share Analysis, By End-user, 2024 and 2035
Figure 46: North America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 47: Europe Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 48: Europe Regulatory Affairs Outsourcing Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 49: Europe Regulatory Affairs Outsourcing Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 50: Europe Regulatory Affairs Outsourcing Market Value Share Analysis, By Service Type, 2024 and 2035
Figure 51: Europe Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Service Type, 2025 to 2035
Figure 52: Europe Regulatory Affairs Outsourcing Market Value Share Analysis, By Product Development Stage, 2024 and 2035
Figure 53: Europe Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Product Development Stage, 2025 to 2035
Figure 54: Europe Regulatory Affairs Outsourcing Market Value Share Analysis, By Enterprise Size, 2024 and 2035
Figure 55: Europe Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Enterprise Size, 2025 to 2035
Figure 56: Europe Regulatory Affairs Outsourcing Market Value Share Analysis, By Therapeutic Area, 2024 and 2035
Figure 57: Europe Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Therapeutic Area, 2025 to 2035
Figure 58: Europe Regulatory Affairs Outsourcing Market Value Share Analysis, By End-user, 2024 and 2035
Figure 59: Europe Regulatory Affairs Outsourcing Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 60: Asia Pacific Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 61: Asia Pacific Regulatory Affairs Outsourcing Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 62: Asia Pacific Regulatory Affairs Outsourcing Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 63: Asia Pacific Regulatory Affairs Outsourcing Market Value Share Analysis, By Service Type, 2024 and 2035
Figure 64: Asia Pacific Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Service Type, 2025 to 2035
Figure 65: Asia Pacific Regulatory Affairs Outsourcing Market Value Share Analysis, By Product Development Stage, 2024 and 2035
Figure 66: Asia Pacific Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Product Development Stage, 2025 to 2035
Figure 67: Asia Pacific Regulatory Affairs Outsourcing Market Value Share Analysis, By Enterprise Size, 2024 and 2035
Figure 68: Asia Pacific Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Enterprise Size, 2025 to 2035
Figure 69: Asia Pacific Regulatory Affairs Outsourcing Market Value Share Analysis, By Therapeutic Area, 2024 and 2035
Figure 70: Asia Pacific Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Therapeutic Area, 2025 to 2035
Figure 71: Asia Pacific Regulatory Affairs Outsourcing Market Value Share Analysis, By End-user, 2024 and 2035
Figure 72: Asia Pacific Regulatory Affairs Outsourcing Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 73: Latin America Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 74: Latin America Regulatory Affairs Outsourcing Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 75: Latin America Regulatory Affairs Outsourcing Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 76: Latin America Regulatory Affairs Outsourcing Market Value Share Analysis, By Service Type, 2024 and 2035
Figure 77: Latin America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Service Type, 2025 to 2035
Figure 78: Latin America Regulatory Affairs Outsourcing Market Value Share Analysis, By Product Development Stage, 2024 and 2035
Figure 79: Latin America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Product Development Stage, 2025 to 2035
Figure 80: Latin America Regulatory Affairs Outsourcing Market Value Share Analysis, By Enterprise Size, 2024 and 2035
Figure 81: Latin America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Enterprise Size, 2025 to 2035
Figure 82: Latin America Regulatory Affairs Outsourcing Market Value Share Analysis, By Therapeutic Area, 2024 and 2035
Figure 83: Latin America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Therapeutic Area, 2025 to 2035
Figure 84: Latin America Regulatory Affairs Outsourcing Market Value Share Analysis, By End-user, 2024 and 2035
Figure 85: Latin America Regulatory Affairs Outsourcing Market Attractiveness Analysis, By End-user, 2025 to 2035
Figure 86: Middle East & Africa Regulatory Affairs Outsourcing Market Value (US$ Bn) Forecast, 2020 to 2035
Figure 87: Middle East & Africa Regulatory Affairs Outsourcing Market Value Share Analysis, by Country/Sub-region, 2024 and 2035
Figure 88: Middle East & Africa Regulatory Affairs Outsourcing Market Attractiveness Analysis, by Country/Sub-region, 2025 to 2035
Figure 89: Middle East & Africa Regulatory Affairs Outsourcing Market Value Share Analysis, By Service Type, 2024 and 2035
Figure 90: Middle East & Africa Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Service Type, 2025 to 2035
Figure 91: Middle East & Africa Regulatory Affairs Outsourcing Market Value Share Analysis, By Product Development Stage, 2024 and 2035
Figure 92: Middle East & Africa Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Product Development Stage, 2025 to 2035
Figure 93: Middle East & Africa Regulatory Affairs Outsourcing Market Value Share Analysis, By Enterprise Size, 2024 and 2035
Figure 94: Middle East & Africa Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Enterprise Size, 2025 to 2035
Figure 95: Middle East & Africa Regulatory Affairs Outsourcing Market Value Share Analysis, By Therapeutic Area, 2024 and 2035
Figure 96: Middle East & Africa Regulatory Affairs Outsourcing Market Attractiveness Analysis, By Therapeutic Area, 2025 to 2035
Figure 97: Middle East & Africa Regulatory Affairs Outsourcing Market Value Share Analysis, By End-user, 2024 and 2035
Figure 98: Middle East & Africa Regulatory Affairs Outsourcing Market Attractiveness Analysis, By End-user, 2025 to 2035