Reports
Reports
The global D-dimer testing market has been pushed by a paradigm change toward demand for next-generation point-of-care D-dimer diagnostics. This is due to the fact that using POC solutions saves waiting times, shortens patient stays, allows for speedier diagnosis, and improves patient satisfaction. The market for D-dimer testing is expected to rise due to the overwhelming benefits received from these solutions in POC settings. The need for D-dimer testing is growing due to an increase in the incidence rate of Venous Thromboembolism (VTE), as well as numerous pulmonary and cardiovascular disorders that create life-threatening difficulties related to blood clot formation.
According to the Centers for Disease Control and Prevention (CDC), approximately 900,000 persons in the U.S. are infected with VTE each year, with around 60,000-100,000 Americans dying as a result. The introduction of aptamers, which can decrease the need for antibodies in D-dimer testing, has shown to be a cost-effective as well as long-lasting method. Aptamers are also less expensive, more stable, and faster to manufacture, as well as having a longer shelf life than antibodies, generating lucrative market potential. Aptamers from Ayass Bioscience, LLC, for example, have been shown to be beneficial in homogenous assays and manual POC agglutination testing.
In numerous clinical diseases, such as coronary artery disease, atrial fibrillation, HIV infection, and acute pancreatitis, comprehensive research investigations have suggested that high D-dimer levels serve as a helpful diagnostic. Systemic coagulation abnormalities, for example, are linked to acute pancreatitis complications. As a result, D-dimer levels can be utilized to estimate the likelihood of acute pancreatitis. These tests are expected to have a broader applicability breadth as a result of these study findings.
The estimated revenue share of reagents and kits category was determined by the frequency with which reagents and kits were purchased, as well as the availability of a substantial stock of ready-to-use reagents. The availability of extremely sensitive automated D-dimer assays that enable safe exclusion of VTE in a short period of time further contributes to the segment's development.
Automated analyzers have gained a lot of traction in the D-dimer testing business in recent years, and they are expected to expand a lot more in the future years. Many studies have found that using these analyzers decreases laboratory turnaround time and speeds up clinical decision-making. Continuous R&D for expanding the application scope of analyzers has a favorable influence on their utilization rate as well.
For instance, Radiometer Limited announced in February 2019 that their AQT90 FLEX analyzers have been successfully evaluated for the D-dimer POCT in potential lower limb deep vein thrombosis patients. The AQT90 FLEX analyzer was implemented and produced findings in less than 21 minutes, significantly enhancing the patient experience. The global D-dimer testing market is projected to be driven by the introduction of new products or the extension of existing products in point-of-care settings.
In the clinical investigation of cardiac and other disorders, the effects of heterophilic antibodies on the calculation of the D-dimer level continue to be a major difficulty. As a result, the outcomes varied significantly. According to studies, combining instrumental approaches, the use of heterophilic antibody blockers, and clinical performance as well as imaging data can clear out interference, indicating the need for more study in this area.
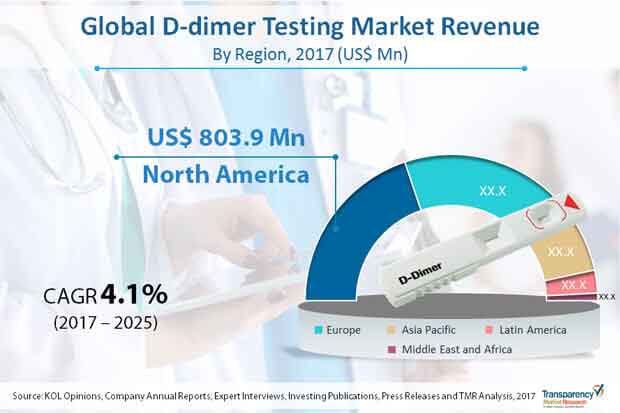
In terms of revenue, North America is predicted to account for a leading position in the global D-dimer testing market during the forecast period. In terms of the number of D-dimer tests conducted globally, Europe is trailed by North America. The primary drivers of the D-dimer market in Europe include growing research activities to increase the specificity of D-dimer tests in order to detect the existence of any coagulation disorders such as PE, DVT, and DIC. Each year, over 600,000 new cases of DVT are detected in the U.S., according to the Society of Interventional Radiology. The Asia Pacific market is likely to be driven by an increase in the number of laboratories, rising demand for D-dimer PoC tests, and the growth of public health care systems. In addition, the D-dimer testing market in the area is likely to be driven by increased interest from significant pharmaceutical companies and a growing population.
The global d-dimer testing market was worth US$ 1.9 bn and north america region is projected to reach a value of US$ 803.9 mn by the end of 2025
D-dimer testing market is anticipated to grow at a CAGR of 4.1% during the forecast period
North America accounted for a major share of the global d-dimer testing market
D-dimer testing market is driven primarily by the rising incidence of pulmonary embolism, deep vein thrombosis, and disseminated intravascular coagulation, for which D-dimer stands as the most popular initial diagnostic test
Key players in the global d-dimer testing market include Thermo Fisher Scientific, Sysmex Corporation, Siemens Healthcare, Helena Biosciences, F. Hoffman-La Roche Ltd., Grifols, S.A., Becton, Dickinson and Company, Abbott Laboratories, Bio/Data Corporation, and Beckman Coulter, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary : Global D-dimer Testing Market
4. Market Overview
4.1. Overview
4.2. Market Indicators
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.3.4. Key Trends
4.4. Global D-dimer Testing Market Analysis and Forecasts, 2015–2025
4.5. Porter's Five Forces Analysis
4.6. D-dimer Testing Market: Value Chain Analysis
5. Global D-dimer Testing Market Analysis and Forecasts, By Testing Method
5.1. Introduction & Definition
5.2. Key Findings / Developments
5.3. Market Value Forecast By Testing Method, 2015–2025
5.3.1. Laboratory
5.3.1.1. Coagulation Analyzers
5.3.1.2. Clinical Chemistry Analyzers
5.3.2. Point-of-Care
5.4. Market Attractiveness By Testing Method
6. Global D-dimer Testing Market Analysis and Forecasts, By Application
6.1. Introduction and Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast By Application, 2015–2025
6.3.1. Deep Vein Thrombosis (DVT)
6.3.2. Pulmonary Embolism (PE)
6.3.3. Disseminated Intravascular Coagulation (DIC)
6.3.4. Others
6.4. Market Attractiveness By Application
7. Global D-dimer Testing Market Analysis and Forecasts, By Region
7.1. Key Findings
7.2. Market Value Share Analysis and Forecast (2015-2025), By Region
7.2.1. North America
7.2.2. Europe
7.2.3. Asia Pacific
7.2.4. Latin America
7.2.5. Middle East & Africa
7.3. Market Attractiveness by Region
8. North America D-dimer Testing Market Analysis and Forecast
8.1. Introduction
8.1.1. Key Findings
8.2. Market Forecast By Testing Method, 2015–2025
8.2.1. Laboratory
8.2.1.1. Coagulation Analyzers
8.2.1.2. Clinical Chemistry Analyzers
8.2.2. Point-of-Care
8.3. Market Forecast By Application, 2015–2025
8.3.1. Deep Vein Thrombosis (DVT)
8.3.2. Pulmonary Embolism (PE)
8.3.3. Disseminated Intravascular Coagulation (DIC)
8.3.4. Others
8.4. Market Forecast By Country, 2015–2025
8.4.1. U.S.
8.4.2. Canada
8.5. Market Attractiveness Analysis
8.5.1. By Testing Method
8.5.2. By Application
8.5.3. By Country
9. Europe D-dimer Testing Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Forecast By Testing Method, 2015–2025
9.2.1. Laboratory
9.2.1.1. Coagulation Analyzers
9.2.1.2. Clinical Chemistry Analyzers
9.2.2. Point-of-Care
9.3. Market Forecast By Application, 2015–2025
9.3.1. Deep Vein Thrombosis (DVT)
9.3.2. Pulmonary Embolism (PE)
9.3.3. Disseminated Intravascular Coagulation (DIC)
9.3.4. Others
9.4. Market Forecast By Country, 2015–2025
9.4.1. Germany
9.4.2. France
9.4.3. U.K.
9.4.4. Spain
9.4.5. Italy
9.4.6. Rest of Europe
9.5. Market Attractiveness Analysis
9.5.1. By Testing Method
9.5.2. By Application
9.5.3. By Country
10. Asia Pacific D-dimer Testing Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Forecast By Testing Method, 2015–2025
10.2.1. Laboratory
10.2.1.1. Coagulation Analyzers
10.2.1.2. Clinical Chemistry Analyzers
10.2.2. Point-of-Care
10.3. Market Forecast By Application, 2015–2025
10.3.1. Deep Vein Thrombosis (DVT)
10.3.2. Pulmonary Embolism (PE)
10.3.3. Disseminated Intravascular Coagulation (DIC)
10.3.4. Others
10.4. Market Forecast By Country, 2015–2025
10.4.1. China
10.4.2. Japan
10.4.3. India
10.4.4. Australia & New Zealand
10.4.5. Rest of Asia Pacific
10.5. Market Attractiveness Analysis
10.5.1. By Testing Method
10.5.2. By Application
10.5.3. By Country
11. Latin America D-dimer Testing Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Forecast By Testing Method, 2015–2025
11.2.1. Laboratory
11.2.1.1. Coagulation Analyzers
11.2.1.2. Clinical Chemistry Analyzers
11.2.2. Point-of-Care
11.3. Market Forecast By Application, 2015–2025
11.3.1. Deep Vein Thrombosis (DVT)
11.3.2. Pulmonary Embolism (PE)
11.3.3. Disseminated Intravascular Coagulation (DIC)
11.3.4. Others
11.4. Market Forecast By Country, 2015–2025
11.4.1. Brazil
11.4.2. Mexico
11.4.3. Rest of Latin America
11.5. Market Attractiveness Analysis
11.5.1. By Testing Method
11.5.2. By Application
11.5.3. By Country
12. Middle East & Africa D-dimer Testing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Forecast By Testing Method, 2015–2025
12.2.1. Laboratory
12.2.1.1. Coagulation Analyzers
12.2.1.2. Clinical Chemistry Analyzers
12.2.2. Point-of-Care
12.3. Market Forecast By Application, 2015–2025
12.3.1. Deep Vein Thrombosis (DVT)
12.3.2. Pulmonary Embolism (PE)
12.3.3. Disseminated Intravascular Coagulation (DIC)
12.3.4. Others
12.4. Market Forecast By Country, 2015–2025
12.4.1. South Africa
12.4.2. GCC Countries
12.4.3. Rest of Middle East & Africa
12.5. Market Attractiveness Analysis
12.5.1. By Testing Method
12.5.2. By Application
12.5.3. By Country
13. Competition Landscape
13.1. D-dimer Testing Market Share Analysis, by Company (2016)
13.2. Company Profiles (Details – Overview, Financials, Recent Developments, Strategy)
13.2.1. Abbott Laboratories
13.2.1.1. Overview (HQ, Employee Strength, Business Segments)
13.2.1.2. Financials
13.2.1.3. Recent Developments
13.2.1.4. SWOT Analysis
13.2.1.5. Strategy
13.2.2. Beckman Coulter, Inc. (Subsidiary of Danaher Corporation)
13.2.2.1. Overview (HQ, Employee Strength, Business Segments)
13.2.2.2. Financials
13.2.2.3. Recent Developments
13.2.2.4. SWOT Analysis
13.2.2.5. Strategy
13.2.3. Grifols, S.A.
13.2.3.1. Overview (HQ, Employee Strength, Business Segments)
13.2.3.2. Financials
13.2.3.3. Recent Developments
13.2.3.4. SWOT Analysis
13.2.3.5. Strategy
13.2.4. Becton, Dickinson and Company
13.2.4.1. Overview (HQ, Employee Strength, Business Segments)
13.2.4.2. Financials
13.2.4.3. Recent Developments
13.2.4.4. SWOT Analysis
13.2.4.5. Strategy
13.2.5. Bio/Data Corporation
13.2.5.1. Overview (HQ, Employee Strength, Business Segments)
13.2.5.2. Recent Developments
13.2.5.3. SWOT Analysis
13.2.5.4. Strategy
13.2.6. F. Hoffmann-La Roche Ltd.
13.2.6.1. Overview (HQ, Employee Strength, Business Segments)
13.2.6.2. Financials
13.2.6.3. Recent Developments
13.2.6.4. SWOT Analysis
13.2.6.5. Strategy
13.2.7. Helena Laboratories Corporation
13.2.7.1. Overview (HQ, Employee Strength, Business Segments)
13.2.7.2. Recent Developments
13.2.7.3. SWOT Analysis
13.2.7.4. Strategy
13.2.8. Sysmex Corporation
13.2.8.1. Overview (HQ, Employee Strength, Business Segments)
13.2.8.2. Financials
13.2.8.3. Recent Developments
13.2.8.4. SWOT Analysis
13.2.8.5. Strategy
13.2.9. Siemens Healthineers (Siemens AG)
13.2.9.1. Overview (HQ, Employee Strength, Business Segments)
13.2.9.2. Financials
13.2.9.3. Recent Developments
13.2.9.4. SWOT Analysis
13.2.9.5. Strategy
13.2.10. Thermo Fisher Scientific, Inc.
13.2.10.1. Overview (HQ, Employee Strength, Business Segments)
13.2.10.2. Financials
13.2.10.3. Recent Developments
13.2.10.4. SWOT Analysis
13.2.10.5. Strategy
List of Figures
Figure 01: D-dimer Testing Market Snapshot
Figure 02: Distribution of D-dimer Testing Market by Region, 2016 and 2025
Figure 03: Global D-dimer Testing Market Size (US$ Mn) Forecast, 2015–2025
Figure 04: Market Value Share, by Testing Method (2016)
Figure 05: Market Value Share, by Application (2016)
Figure 06: Global D-dimer Testing Market Value Share Analysis, by Testing Method, 2016 and 2025
Figure 07: Global D-dimer Testing Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Point-of-care, 2015–2025
Figure 08: Global D-dimer Testing Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Laboratory, 2015–2025
Figure 09: Global D-dimer Testing Market Attractiveness Analysis, by Testing Method, 2017–2025
Figure 10: Global D-dimer Testing Market Value Share Analysis, by Application, 2016 and 2025
Figure 11: Global D-dimer Testing Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Deep Vein Thrombosis (DVT), 2015–2025
Figure 12: Global D-dimer Testing Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Pulmonary Embolism (PE), 2015–2025
Figure 13: Global D-dimer Testing Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Disseminated Intravascular Coagulation (DIC), 2015–2025
Figure 14: Global D-dimer Testing Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2015–2025
Figure 15: Global D-dimer Testing Market Attractiveness Analysis, by Application, 2017–2025
Figure 16: Global D-dimer Testing Market Value Share Analysis, by Region, 2016 and 2025
Figure 17: Global D-dimer testing Market Attractiveness Analysis, by Region
Figure 18: North America D-dimer Testing Market Size (US$ Mn) Forecast, 2017–2025
Figure 19: North America D-dimer Testing Market Attractiveness, by Country
Figure 20: North America D-dimer Testing Market Value Share, by Testing Method, 2016 and 2025
Figure 21: North America D-dimer Testing Market Value Share, by Application, 2016 and 2025
Figure 22: North America D-dimer Testing Market Value Share, by Country, 2016 and 2025
Figure 23: North America D-dimer Testing Market Attractiveness, by Testing Method
Figure 24: North America D-dimer Testing Market Attractiveness, by Application
Figure 25: Europe D-dimer Testing Market Size (US$ Mn) Forecast, 2017–2025
Figure 26: Europe D-dimer Testing Market Attractiveness Analysis, by Country/Sub-region
Figure 27: Europe D-dimer Testing Market Value Share Analysis, by Testing Method, 2016 and 2025
Figure 28: Europe D-dimer Testing Market Value Share Analysis, by Application, 2016 and 2025
Figure 29: Europe D-dimer Testing Market Value Share Analysis, by Country/Sub-region, 2016 and 2025
Figure 30: Europe D-dimer Testing Market Attractiveness Analysis, by Testing Method
Figure 31: Europe D-dimer Testing Market Attractiveness Analysis, by Application
Figure 32: Asia Pacific D-dimer Testing Market Size (US$ Mn) Forecast, 2017–2025
Figure 33: Asia Pacific Market Attractiveness, by Country/Sub-region
Figure 34: Asia Pacific D-dimer Testing Market Value Share, by Testing Method, 2016 and 2025
Figure 35: Asia Pacific D-dimer Testing Market Value Share, by Application, 2016 and 2025
Figure 36: Asia Pacific D-dimer Testing Market Value Share, by Country/Sub-region, 2016 and 2025
Figure 37: Asia Pacific D-dimer Testing Market Attractiveness, by Testing Method
Figure 38: Asia Pacific D-dimer Testing Market Attractiveness, by Application
Figure 39: Latin America D-dimer Testing Market Size (US$ Mn) Forecast, 2017–2025
Figure 40: Latin America D-dimer Testing Market Attractiveness Analysis, by Country/Sub-region
Figure 41: Latin America D-dimer Testing Market Value Share Analysis, by Testing Method, 2016 and 2025
Figure 42: Latin America D-dimer Testing Market Value Share Analysis, by Application, 2016 and 2025
Figure 43: Latin America D-dimer Testing Market Value Share Analysis, by Country/Sub-region, 2016 and 2025
Figure 44: Latin America D-dimer Testing Market Attractiveness Analysis, by Testing Method
Figure 45: Latin America D-dimer Testing Market Attractiveness Analysis, by Application
Figure 46: Middle East & Africa D-dimer Testing Market Size (US$ Mn) Forecast, 2017–2025
Figure 47: Middle East & Africa D-dimer Testing Market Attractiveness, by Country/Sub-region
Figure 48: Middle East & Africa D-dimer Testing Market Value Share, by Testing Method, 2016 and 2025
Figure 49: Middle East & Africa D-dimer Testing Market Value Share, by Application, 2016 and 2025
Figure 50: Middle East & Africa D-dimer Testing Market Value Share, by Country/Sub-region, 2016 and 2025
Figure 51: Middle East & Africa D-dimer Testing Market Attractiveness, by Testing Method
Figure 52: Middle East & Africa D-dimer Testing Market Attractiveness, by Application
Figure 53: Global D-dimer Testing Market Share Analysis, by Company (2016)
Figure 54: Abbott Laboratories Revenue (US$ Mn) and Y-o-Y Growth (%), 2013–2016
Figure 55: Abbott Laboratories Breakdown of Net Sales (%), by Region/Country, 2016
Figure 56: Abbott Laboratories Breakdown of Net Sales (%), by Business Segment, 2016
Figure 57: Abbott Laboratories R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2013–2016
Figure 58: Danaher’s Diagnostics Business Segment Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2016
Figure 59: Danaher’s Diagnostics Business Segment R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2014–2016
Figure 60: Danaher’s Diagnostics Business Segment Net Sales, by Region, 2016
Figure 61: Breakdown of Net Sales (%), Danaher’s Diagnostics Business Segment, by Product, 2016
Figure 62: Grifols, S.A. Revenue (US$ Mn) & Y-o-Y Growth (%), 2013–2016
Figure 63: Grifols, S.A. Research & Development Expenses, 2014–2016
Figure 64: Grifols, S.A. Breakdown of Net Sales, by Business Segment, 2016
Figure 65: Grifols, S.A. Breakdown of Net Sales, by Region, 2016
Figure 66: Becton, Dickinson and Company Revenue (US$ Mn) and Y-o-Y Growth (%), 2013–2016
Figure 67: Becton, Dickinson and Company R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2013–2016
Figure 68: Becton, Dickinson and Company Breakdown of Net Sales, by Region, 2016
Figure 69: Becton, Dickinson and Company Breakdown of Net Sales, by Business Segment, 2016
Figure 70: F. Hoffmann-La Roche Ltd. Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2017
Figure 71: F. Hoffmann-La Roche Ltd. R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2014–2017
Figure 72: F. Hoffmann-La Roche Ltd. Breakdown of Net Sales, by Region, 2016
Figure 73: F. Hoffmann-La Roche Ltd. Breakdown of Net Sales, by Business Segment, 2016
Figure 74: Sysmex Corporation Revenue (US$ Mn) and Y-o-Y Growth (%), 2014–2017
Figure 75: Sysmex Corporation Breakdown of Net Sales, by Company Product Type, 2017
Figure 76: Sysmex Corporation Breakdown of Net Sales, by Business Segment, 2017
Figure 77: Sysmex Corporation Breakdown of Net Sales, by Region, 2017
Figure 78: Siemens Healthineers Revenue (US$ Mn) and Y-o-Y Growth (%), 2013–2016
Figure 79: Siemens Healthineers Diagnostics Segment R&D Expenses (US$ Mn) and Y-o-Y Growth (%), 2015–2017
Figure 80: Siemens Healthineers Diagnostics Business Segment, Breakdown of Net Sales (%), by Region, 2016
Figure 81: Siemens Healthineers Diagnostics Business Segment, Breakdown of Net Sales (%), by Business Segment, 2016
Figure 82: Thermo Fisher Scientific, Inc. Revenue (US$ Bn) and Y-o-Y Growth (%), 2013–2016
Figure 83: Thermo Fisher Scientific, Inc. Breakdown of Net Sales, by Region, 2016
Figure 84: Thermo Fisher Scientific, Inc. Breakdown of Net Sales, by Business Segment, 2016
Figure 85: Thermo Fisher Scientific, Inc. R&D Expense (US$ Mn) and Y-o-Y Growth (%), 2013–2016