Reports
Reports
The global cell free RNA diagnostics market size was valued at US$ 46.1 million in 2024 and is projected to reach US$ 204.5 million by 2035, expanding at a CAGR of 14.5% from 2025 to 2035. The market growth is driven by advancements in sequencing technologies and growing demand for non-invasive testing methods.
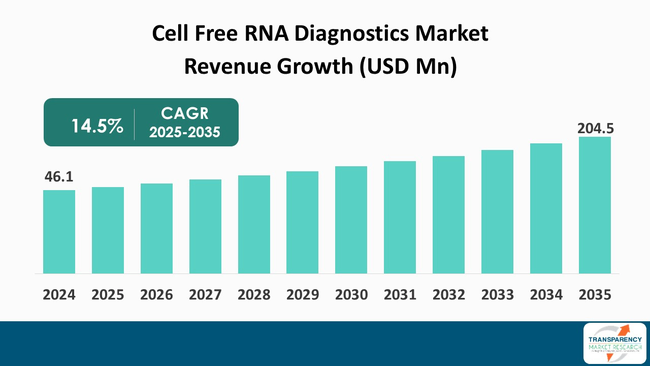
The cell free RNA (cfRNA) diagnostics market is set to grow substantially. This growth is largely attributed to the progress made in molecular biology and the ongoing trend toward less invasive testing methods. cfRNA, which is a nucleic acid that can be found in different physiological fluids like blood and urine, is a rich source of information about the diseased states, especially cancer and prenatal testing.
On top of that, the increasing prevalence of chronic diseases is a major demand driver for innovative diagnostic solutions. In addition, the elderly population is also increasing, hence their demand for diagnostic solutions. Besides, tech advances in sequencing and bioinformatics are vastly improving the sensitivity and specificity of cfRNA assays.
Since healthcare systems are progressively implementing precision medicine strategies, the use of cfRNA diagnostics in the day-to-day clinical routine will, most likely, be accelerated.
However, issues such as regulatory challenges, standardization of testing protocols, and the requirement for substantial investment in research and development activities may limit the growth. Yet, the partnership between biotech companies and research institutions and the increasing support for cfRNA research are some of the telltale signs that this market has a bright future ahead. The market for cfRNA diagnostics is, in essence, a major shift in the way diseases are diagnosed and managed.
Cell free RNA (cfRNA) diagnostics is a type of molecular diagnostics, which makes it is possible to analyze the RNA molecules present in the body fluids like blood and urine. This noninvasive method is an important source of information about different kinds of diseases, especially in cancer, which can be detected, its progression can be monitored, and the treatment response can be evaluated through oncology-related cfRNA applications.
With the help of cutting-edge sequencing technologies and bioinformatics, the sensitivity and specificity of cfRNA assays have been improved to a great extent, thus making them more dependable for clinical use. This is why cfRNA has become popular for use in prenatal testing, infectious diseases, and monitoring of autoimmune disorders. Besides, as the medical world is moving toward personalized medicine, the significance of cfRNA diagnostics in determining and adjusting therapeutic strategies is growing day by day.
Though such enormous potential was shown, the development of this field has been restrained by the requirement for standardized protocols and the procedure for getting regulatory approval. However, the commitment to research and the collaboration between academic institutions and biotech companies will, undoubtedly, fuel innovation and leverage the use of cfRNA diagnostics to close more clinical needs, hence promoting patient outcomes and changing disease management strategies.
| Attribute | Detail |
|---|---|
| Cell Free RNA Diagnostics Market Drivers |
|
Advancements in sequencing technologies represent a major factor enabling the expansion of the cell free RNA (cfRNA) diagnostics market. They have considerably improved the capability of extracting and analyzing cfRNA from different biological fluids. These state-of-the-art technologies such as next-generation sequencing (NGS) and single-cell sequencing offer fast, reliable, and relatively inexpensive methods of locating disease-associated RNA biomarker, especially in cancer.
By continuously improving sequencing technologies, researchers and doctors are given the tools to deeply understand the complex nature of cfRNA profiles, thus enabling early diagnosis and patient-specific therapy. The adoption of precision medicine by healthcare systems will be a great advantage to implementing advanced sequencing technologies into cfRNA diagnostics, which is at the forefront of a patient care revolution with timely intervention and better prognosis.
The growing demand for non-invasive testing methods is a notable driver of the cell free RNA (cfRNA) diagnostics market. Healthcare providers and patients increasingly prefer tests that minimize physical discomfort and risk, thereby making liquid biopsies that analyze cfRNA from blood and the other bodily fluids an attractive option. These non-invasive approaches eliminate the requirement of more invasive procedures such as biopsies, which can lead to complications and need longer recovery times.
Non-invasive testing methods are especially appealing in oncology, where early detection as well as monitoring of cancer progression are crucial. cfRNA analysis provides vital information about tumor dynamics and is capable of detecting the presence of malignancies at an earlier stage. This ability of gathering critical insights without invasive interventions not only improves patient experience but also compliance with screening protocols.
Moreover, the rising incidences of chronic diseases and the focus on preventive healthcare further drive the demand for non-invasive diagnostics. As healthcare systems worldwide prioritize patient-centered approaches, adopting cfRNA diagnostics is on the rise. This trend aligns with advancements in technology, thereby increasing the reliability and precision of non-invasive tests, ultimately leading to better patient outcomes.
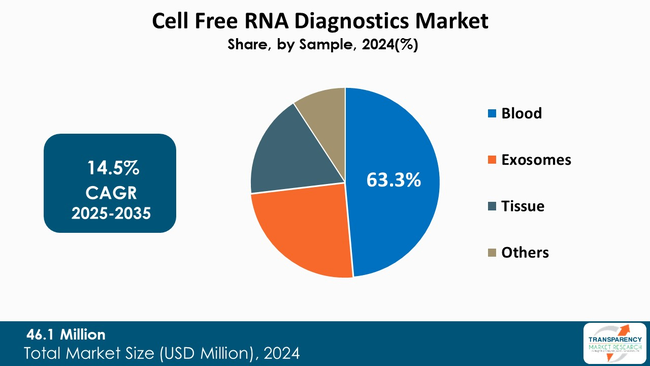
Blood sample section of the market is the major factor for growth in cell free RNA (cfRNA) diagnostics market due to the fact that it is comfortable and effective to give the necessary health information.
Besides, blood samples are endowed with a diverse range of cfRNA that can reveal a lot of information on disease states with emphasis on cancer and infectious diseases. By targeting cfRNA in blood, cancers can be detected at their earliest stages, and responses to treatment can also be monitored, thus, making personalized medicine strategies more efficient. The uptake of cfRNA clinical practice is skyrocketing as research findings continue to confirm the incredible diagnostic potential of this nucleic acid isolated from blood.
In addition, the rise of chronic diseases and a paradigm shift to preventive healthcare are some of the factors that have triggered the increase of this segment. As healthcare becomes more system-based and focuses on early diagnosis and timely intervention, the need for reliable and efficient blood-based tests is escalating. This movement together with the technological advancements in sequencing and bioinformatics makes the blood sample segment a major player in the cfRNA diagnostics market that is changing.
| Attribute | Detail |
|---|---|
| Leading Region |
|
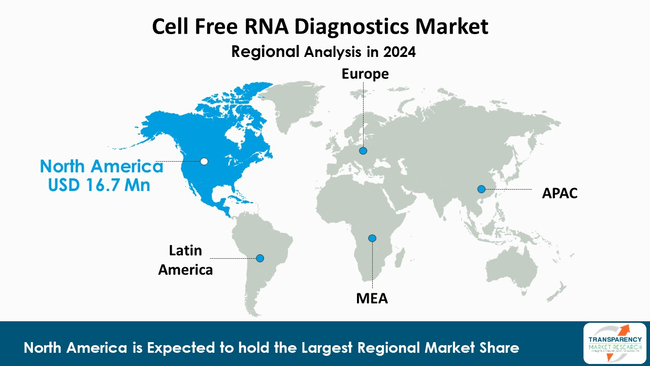
North America is at the forefront of the cell free RNA (cfRNA) diagnostics market, holding the largest revenue share of 36.2%, mainly due to a well-established healthcare system, large investments in research and development, and a strong focus on precision medicine. The region hosts the major biotech and pharmaceutical companies in the world, which are the primary sources of innovations in cfRNA technologies. Such a vibrant ecosystem encourages the partnership of the academic and industrial sectors, thus the findings from the research are quickly applied to clinical practice.
Moreover, North America is characterized by a high level of consciousness and demand for sophisticated diagnostic tools that is shared by healthcare providers and patients alike. The rise in the incidences of lifestyle diseases, cancer in particular, has led to the need for less invasive and more efficient diagnostic methods, so cfRNA diagnostics have become appealing.
In addition, the existence of well-established healthcare reimbursement models is one of the main reasons why the adoption of state-of-the-art diagnostics is not a problem. As healthcare systems shift their focus toward early detection and personalized treatment regimens, the use of cfRNA diagnostics will be on the rise. Such a mix of factors makes the North American market leader the cfRNA diagnostics market, thus the region is leading the way with continuous advancements and better patient outcomes.
Agilent Technologies, Inc., AMS Biotechnology (Europe) Ltd., Bio-Rad Laboratories, Inc., BioVision, Inc., Canopy Biosciences, LLC, F. Hoffmann-La Roche Ltd., Geneaid Biotech Ltd., Illumina, Inc., QIAGEN N.V., Lexogen GmbH, LGC Biosearch Technologies, Merck KGaA, New England Biolabs, Inc., Norgen Biotek Corp., Omega Bio-Tek, Inc., PerkinElmer, Inc. are the key players governing the global cell free RNA diagnostics market.
Each of these players has been profiled in the cell free RNA diagnostics market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2024 | US$ 46.1 Mn |
| Forecast Value in 2035 | More than US$ 204.5 Mn |
| CAGR | 14.5% |
| Forecast Period | 2025-2035 |
| Historical Data Available for | 2020-2023 |
| Quantitative Units | US$ Mn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation | By Product Type
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 46.1 Mn in 2024
It is projected to cross US$ 204.5 Mn by the end of 2035
Advancements in sequencing technologies and growing demand for non-invasive testing methods
It is anticipated to grow at a CAGR of 14.5% from 2025 to 2035
North America is expected to account for the largest share from 2025 to 2035
Agilent Technologies, Inc., AMS Biotechnology (Europe) Ltd., Bio-Rad Laboratories, Inc., BioVision, Inc., Canopy Biosciences, LLC, F. Hoffmann-La Roche Ltd., Geneaid Biotech Ltd., Illumina, Inc., QIAGEN N.V., Lexogen GmbH, LGC Biosearch Technologies, Merck KGaA, New England Biolabs, Inc., Norgen Biotek Corp., Omega Bio-Tek, Inc., PerkinElmer, Inc.
Table 01: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2020 to 2035
Table 02: Global Cell Free RNA Diagnostics Market Value (US$ Mn), by Sample Collection, 2020 to 2035
Table 03: Global Cell Free RNA Diagnostics Market Value (US$ Mn), by Isolation, 2020 to 2035
Table 04: Global Cell Free RNA Diagnostics Market Value (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Table 05: Global Cell Free RNA Diagnostics Market Value (US$ Mn), by Bioinformatics Analysis Platforms, 2020 to 2035
Table 06: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Sample, 2020 to 2035
Table 07: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Application, 2020 to 2035
Table 08: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Technology, 2020 to 2035
Table 09: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2020 to 2035
Table 10: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Region, 2020 to 2035
Table 11: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2020 to 2035
Table 12: North America Cell Free RNA Diagnostics Market Value (US$ Mn), by Sample Collection, 2020 to 2035
Table 13: North America Cell Free RNA Diagnostics Market Value (US$ Mn), by Isolation, 2020 to 2035
Table 14: North America Cell Free RNA Diagnostics Market Value (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Table 15: North America Cell Free RNA Diagnostics Market Value (US$ Mn), by Bioinformatics Analysis Platforms, 2020 to 2035
Table 16: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Sample, 2020 to 2035
Table 17: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Application, 2020 to 2035
Table 18: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Technology, 2020 to 2035
Table 19: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2020 to 2035
Table 20: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Region, 2020 to 2035
Table 21: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2020 to 2035
Table 22: Europe Cell Free RNA Diagnostics Market Value (US$ Mn), by Sample Collection, 2020 to 2035
Table 23: Europe Cell Free RNA Diagnostics Market Value (US$ Mn), by Isolation, 2020 to 2035
Table 24: Europe Cell Free RNA Diagnostics Market Value (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Table 25: Europe Cell Free RNA Diagnostics Market Value (US$ Mn), by Bioinformatics Analysis Platforms, 2020 to 2035
Table 26: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Sample, 2020 to 2035
Table 27: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Application, 2020 to 2035
Table 28: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Technology, 2020 to 2035
Table 29: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2020 to 2035
Table 30: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Region, 2020 to 2035
Table 31: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2020 to 2035
Table 32: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn), by Sample Collection, 2020 to 2035
Table 33: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn), by Isolation, 2020 to 2035
Table 34: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Table 35: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn), by Bioinformatics Analysis Platforms, 2020 to 2035
Table 36: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Sample, 2020 to 2035
Table 37: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Application, 2020 to 2035
Table 38: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Technology, 2020 to 2035
Table 39: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2020 to 2035
Table 40: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Region, 2020 to 2035
Table 41: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2020 to 2035
Table 42: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn), by Sample Collection, 2020 to 2035
Table 43: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn), by Isolation, 2020 to 2035
Table 44: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Table 45: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn), by Bioinformatics Analysis Platforms, 2020 to 2035
Table 46: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Sample, 2020 to 2035
Table 47: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Application, 2020 to 2035
Table 48: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Technology, 2020 to 2035
Table 49: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2020 to 2035
Table 50: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Region, 2020 to 2035
Table 51: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2020 to 2035
Table 52: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn), by Sample Collection, 2020 to 2035
Table 53: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn), by Isolation, 2020 to 2035
Table 54: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Table 55: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn), by Bioinformatics Analysis Platforms, 2020 to 2035
Table 56: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Sample, 2020 to 2035
Table 57: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Application, 2020 to 2035
Table 58: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Technology, 2020 to 2035
Table 59: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2020 to 2035
Table 60: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, by Region, 2020 to 2035
Figure 01: Global Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 02: Global Cell Free RNA Diagnostics Market Value Share Analysis, by Product Type, 2024 and 2035
Figure 03: Global Cell Free RNA Diagnostics Market Attractiveness Analysis, by Product Type, 2025 to 2035
Figure 04: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Sample Collection, 2020 to 2035
Figure 05: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Isolation, 2020 to 2035
Figure 06: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Library Preparation and Sequencing, 2020 to 2035
Figure 07: Global Cell Free RNA Diagnostics Market Value Share Analysis, by Sample, 2024 and 2035
Figure 08: Global Cell Free RNA Diagnostics Market Attractiveness Analysis, by Sample, 2025 to 2035
Figure 09: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Blood, 2020 to 2035
Figure 10: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Exosomes, 2020 to 2035
Figure 11: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Tissue, 2020 to 2035
Figure 12: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Others, 2020 to 2035
Figure 13: Global Cell Free RNA Diagnostics Market Value Share Analysis, by Application, 2024 and 2035
Figure 14: Global Cell Free RNA Diagnostics Market Attractiveness Analysis, by Application, 2025 to 2035
Figure 15: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Cancer, 2020 to 2035
Figure 16: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Genetic Disorder, 2020 to 2035
Figure 17: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Metabolic Disorder, 2020 to 2035
Figure 18: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Infectious Disease, 2020 to 2035
Figure 19: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Prenatal Screening, 2020 to 2035
Figure 20: Global Cell Free RNA Diagnostics Market Value Share Analysis, by Technology, 2024 and 2035
Figure 21: Global Cell Free RNA Diagnostics Market Attractiveness Analysis, by Technology, 2025 to 2035
Figure 22: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by PCR Based Method (ddPCR, qPCR), 2020 to 2035
Figure 23: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Next Generation Sequencing, 2020 to 2035
Figure 24: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Others, 2020 to 2035
Figure 25: Global Cell Free RNA Diagnostics Market Value Share Analysis, by End-user, 2024 and 2035
Figure 26: Global Cell Free RNA Diagnostics Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 27: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Hospitals & clinics, 2020 to 2035
Figure 28: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Diagnostic Laboratories, 2020 to 2035
Figure 29: Global Cell Free RNA Diagnostics Market Revenue (US$ Mn), by Others, 2020 to 2035
Figure 30: Global Cell Free RNA Diagnostics Market Value Share Analysis, by Region, 2024 and 2035
Figure 31: Global Cell Free RNA Diagnostics Market Attractiveness Analysis, by Region, 2025 to 2035
Figure 32: North America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 33: North America Cell Free RNA Diagnostics Market Value Share Analysis, by Product Type, 2024 and 2035
Figure 34: North America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Product Type, 2025 to 2035
Figure 35: North America Cell Free RNA Diagnostics Market Value Share Analysis, by Sample, 2024 and 2035
Figure 36: North America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Sample, 2025 to 2035
Figure 37: North America Cell Free RNA Diagnostics Market Value Share Analysis, by Application, 2024 and 2035
Figure 38: North America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Application, 2025 to 2035
Figure 39: North America Cell Free RNA Diagnostics Market Value Share Analysis, by Technology, 2024 and 2035
Figure 40: North America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Technology, 2025 to 2035
Figure 41: North America Cell Free RNA Diagnostics Market Value Share Analysis, by End-user, 2024 and 2035
Figure 42: North America Cell Free RNA Diagnostics Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 43: North America Cell Free RNA Diagnostics Market Value Share Analysis, by Region, 2024 and 2035
Figure 44: North America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Region, 2025 to 2035
Figure 45: Europe Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 46: Europe Cell Free RNA Diagnostics Market Value Share Analysis, by Product Type, 2024 and 2035
Figure 47: Europe Cell Free RNA Diagnostics Market Attractiveness Analysis, by Product Type, 2025 to 2035
Figure 48: Europe Cell Free RNA Diagnostics Market Value Share Analysis, by Sample, 2024 and 2035
Figure 49: Europe Cell Free RNA Diagnostics Market Attractiveness Analysis, by Sample, 2025 to 2035
Figure 50: Europe Cell Free RNA Diagnostics Market Value Share Analysis, by Application, 2024 and 2035
Figure 51: Europe Cell Free RNA Diagnostics Market Attractiveness Analysis, by Application, 2025 to 2035
Figure 52: Europe Cell Free RNA Diagnostics Market Value Share Analysis, by Technology, 2024 and 2035
Figure 53: Europe Cell Free RNA Diagnostics Market Attractiveness Analysis, by Technology, 2025 to 2035
Figure 54: Europe Cell Free RNA Diagnostics Market Value Share Analysis, by End-user, 2024 and 2035
Figure 55: Europe Cell Free RNA Diagnostics Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 56: Europe Cell Free RNA Diagnostics Market Value Share Analysis, by Region, 2024 and 2035
Figure 57: Europe Cell Free RNA Diagnostics Market Attractiveness Analysis, by Region, 2025 to 2035
Figure 58: Asia Pacific Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 59: Asia Pacific Cell Free RNA Diagnostics Market Value Share Analysis, by Product Type, 2024 and 2035
Figure 60: Asia Pacific Cell Free RNA Diagnostics Market Attractiveness Analysis, by Product Type, 2025 to 2035
Figure 61: Asia Pacific Cell Free RNA Diagnostics Market Value Share Analysis, by Sample, 2024 and 2035
Figure 62: Asia Pacific Cell Free RNA Diagnostics Market Attractiveness Analysis, by Sample, 2025 to 2035
Figure 63: Asia Pacific Cell Free RNA Diagnostics Market Value Share Analysis, by Application, 2024 and 2035
Figure 64: Asia Pacific Cell Free RNA Diagnostics Market Attractiveness Analysis, by Application, 2025 to 2035
Figure 65: Asia Pacific Cell Free RNA Diagnostics Market Value Share Analysis, by Technology, 2024 and 2035
Figure 66: Asia Pacific Cell Free RNA Diagnostics Market Attractiveness Analysis, by Technology, 2025 to 2035
Figure 67: Asia Pacific Cell Free RNA Diagnostics Market Value Share Analysis, by End-user, 2024 and 2035
Figure 68: Asia Pacific Cell Free RNA Diagnostics Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 69: Asia Pacific Cell Free RNA Diagnostics Market Value Share Analysis, by Region, 2024 and 2035
Figure 70: Asia Pacific Cell Free RNA Diagnostics Market Attractiveness Analysis, by Region, 2025 to 2035
Figure 71: Latin America Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 72: Latin America Cell Free RNA Diagnostics Market Value Share Analysis, by Product Type, 2024 and 2035
Figure 73: Latin America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Product Type, 2025 to 2035
Figure 74: Latin America Cell Free RNA Diagnostics Market Value Share Analysis, by Sample, 2024 and 2035
Figure 75: Latin America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Sample, 2025 to 2035
Figure 76: Latin America Cell Free RNA Diagnostics Market Value Share Analysis, by Application, 2024 and 2035
Figure 77: Latin America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Application, 2025 to 2035
Figure 78: Latin America Cell Free RNA Diagnostics Market Value Share Analysis, by Technology, 2024 and 2035
Figure 79: Latin America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Technology, 2025 to 2035
Figure 80: Latin America Cell Free RNA Diagnostics Market Value Share Analysis, by End-user, 2024 and 2035
Figure 81: Latin America Cell Free RNA Diagnostics Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 82: Latin America Cell Free RNA Diagnostics Market Value Share Analysis, by Region, 2024 and 2035
Figure 83: Latin America Cell Free RNA Diagnostics Market Attractiveness Analysis, by Region, 2025 to 2035
Figure 84: Middle East and Africa Cell Free RNA Diagnostics Market Value (US$ Mn) Forecast, 2020 to 2035
Figure 85: Middle East and Africa Cell Free RNA Diagnostics Market Value Share Analysis, by Product Type, 2024 and 2035
Figure 86: Middle East and Africa Cell Free RNA Diagnostics Market Attractiveness Analysis, by Product Type, 2025 to 2035
Figure 87: Middle East and Africa Cell Free RNA Diagnostics Market Value Share Analysis, by Sample, 2024 and 2035
Figure 88: Middle East and Africa Cell Free RNA Diagnostics Market Attractiveness Analysis, by Sample, 2025 to 2035
Figure 89: Middle East and Africa Cell Free RNA Diagnostics Market Value Share Analysis, by Application, 2024 and 2035
Figure 90: Middle East and Africa Cell Free RNA Diagnostics Market Attractiveness Analysis, by Application, 2025 to 2035
Figure 91: Middle East and Africa Cell Free RNA Diagnostics Market Value Share Analysis, by Technology, 2024 and 2035
Figure 92: Middle East and Africa Cell Free RNA Diagnostics Market Attractiveness Analysis, by Technology, 2025 to 2035
Figure 93: Middle East and Africa Cell Free RNA Diagnostics Market Value Share Analysis, by End-user, 2024 and 2035
Figure 94: Middle East and Africa Cell Free RNA Diagnostics Market Attractiveness Analysis, by End-user, 2025 to 2035
Figure 95: Middle East and Africa Cell Free RNA Diagnostics Market Value Share Analysis, by Region, 2024 and 2035
Figure 96: Middle East and Africa Cell Free RNA Diagnostics Market Attractiveness Analysis, by Region, 2025 to 2035