Reports
Reports
Analysts’ Viewpoint
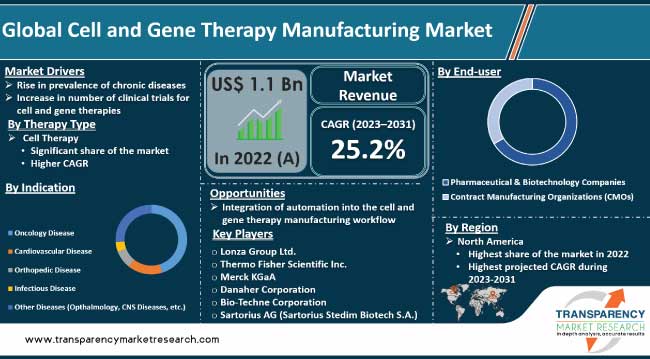
Scientific advancements and growing understanding of cellular & genetic mechanisms are driving the global cell and gene therapy manufacturing market. Cell and gene therapies offer tailored interventions that target the root cause of diseases at a cellular level. Advancements in manufacturing technologies and processes are enabling improved scalability and cost-effectiveness in the production of these therapies. Innovations such as automated bioreactors, closed-system processing, and novel gene delivery techniques are expected to propel the global cell and gene therapy manufacturing market size during the forecast period.
Approval of cell and gene therapies by regulatory agencies offers lucrative opportunities to market players. Pharmaceutical and biotechnology companies are entering into strategic collaborations to accelerate research, development, and commercialization of cell and gene therapies in order to increase market revenue and share.
Cell and gene therapy manufacturing stands at the forefront of medical innovation, and is poised to revolutionize the treatment landscape for various diseases. This cutting-edge field harnesses the power of cellular and genetic engineering to develop personalized and targeted therapies, holding immense promise for addressing unmet medical needs.
Unlike traditional pharmaceuticals, these therapies involve manipulating patients' own cells or genes to combat diseases at their root causes. Convergence of advanced biotechnology, precision medicine, and manufacturing expertise has paved the way for these therapies to become a reality, with remarkable successes observed in conditions such as cancer and genetic disorders.
Rise in prevalence of chronic diseases is fueling the cell and gene therapy manufacturing market growth. Chronic diseases, such as cancer, cardiovascular disorders, genetic anomalies, and autoimmune diseases, continue to burden healthcare systems across the world. According to the World Health Organization (WHO), 60% of adults globally have at least one chronic disease. This number is expected to rise to 70% by 2025.
Cell and gene therapies offer a revolutionary approach by utilizing patients' own cells or modifying their genetic makeup to provide personalized and targeted treatments. This paradigm shift from traditional therapies has spurred research and investment in manufacturing technologies to develop, scale, and deliver these advanced therapies to a wider population.
Rise in demand for such therapies has spurred collaborations between biotechnology firms, pharmaceutical companies, and research institutions to optimize production processes and ensure the delivery of safe and effective treatments. Consequently, the market has witnessed a surge in funding, technological advancements, and regulatory support to facilitate the development and commercialization of these therapies.
Global cell and gene therapy manufacturing market demand is poised for sustained expansion as chronic diseases continue to affect a larger proportion of the global population.
The cell and gene therapy manufacturing market is witnessing accelerated growth, driving the availability and affordability of these therapies for patients globally. The influx of funds signifies the industry's commitment to revolutionize medical treatment through personalized and targeted approaches. This offers hope for patients with challenging medical conditions.
The market is experiencing robust growth due to substantial investment by pharmaceutical and biotechnology companies. These investments play a pivotal role in advancing the field of regenerative medicine and personalized therapies.
The U.S. Government is the largest single investor in pharmaceutical and biotechnology research and development, providing over US$ 40 Bn annually. The FDA also provides funding for research, particularly in areas related to drug safety and efficacy.
Pharmaceutical and biotech firms are recognizing the transformative potential of cell and gene therapies to address previously untreatable diseases. Consequently, they are committing substantial resources to research, development, and production of these therapies. This financial support has enabled the expansion of manufacturing capabilities, leading to an increase in efficiency and scalability in producing these complex therapies.
Investments are being directed toward cutting-edge technologies, process optimization, and infrastructure development to overcome challenges associated with manufacturing cell and gene therapies. Automation, advanced bioreactors, and innovative techniques are being integrated to ensure consistent and high-quality production. These investments also aid in meeting regulatory requirements, which are crucial for bringing these therapies to market.
In terms of product type, the consumables segment accounted for the largest global cell and gene therapy manufacturing market share in 2022. This is ascribed to increase in demand for consumables, such as culture media and reagents, in the development of cell and gene therapies.
The combination of convenience, reliability, and the unique requirements of the cell and gene therapy manufacturing process contributed to the prominent share of the consumables segment.
Advancements in manufacturing technologies and regulatory compliance emphasize quality consumables. These factors collectively amplify the consumables segment's pivotal role in shaping the market's landscape.
The trend toward single-use technologies and increase in demand for cell and gene therapies are propelling the growth and dominance of the segment over equipment, software, and systems.
Based on indication, the oncology segment is poised to dominate the global market during the forecast period. Convergence of advanced biotechnology, personalized medicine, and innovative treatment approaches has propelled oncology to the forefront of this market.
Cell and gene therapies have demonstrated remarkable potential in revolutionizing cancer treatment, offering precise and targeted interventions that address the complexity and heterogeneity of various malignancies.
The unique challenges posed by cancer, coupled with the remarkable progress in understanding genetic and cellular mechanisms, have led to the development of cutting-edge therapies. CAR-T (Chimeric Antigen Receptor T-cell) therapies, for instance, have gained significant attention for their success in treating certain types of leukemia and lymphoma. These therapies involve modifying a patient's own T-cells to recognize and destroy cancer cells with unprecedented specificity.
The versatility of cell and gene therapies in targeting specific mutations, harnessing the immune system, and enhancing the body's natural defense mechanisms holds immense promise for creating personalized treatment regimens. Consequently, investment and research efforts in oncology-related cell and gene therapy manufacturing have surged, creating a dynamic landscape of collaborations, clinical trials, and commercial ventures.
In conclusion, the profound impact of cell and gene therapies on oncology, along with growing investment in research, development, and manufacturing, are expected to consolidate oncology's significant share in the market.
As these therapies continue to demonstrate their potential in addressing unmet medical needs and improving patient outcomes, the oncology segment is set to play a pivotal role in shaping the future of medicine.
In terms of end-user, the pharmaceutical & biotechnology companies segment is projected to account for the leading share of the global market during the forecast period. Pharmaceutical & biotechnology companies' extensive resources, research expertise, and established infrastructure uniquely position them to drive innovation, scale up production, and bring these transformative therapies to a broader patient population.
Increase in demand for personalized and targeted treatments has induced pharmaceutical and biotech companies to focus on developing and commercializing cell and gene therapies. Strategic integration of these therapies into their portfolios allows these companies to diversify their offerings and tap into a burgeoning market that addresses previously unmet medical needs.
These companies possess the capabilities to navigate the complex regulatory landscape and ensure compliance with stringent manufacturing requirements. Their experience in bringing traditional pharmaceuticals to market lends credibility and expertise to the development, manufacturing, and distribution of cell and gene therapies.
Collaborations and partnerships between pharmaceutical and biotechnology firms and academic institutions, research organizations, and manufacturing experts are also on the rise. These collaborations facilitate knowledge exchange, technology transfer, and accelerated development timelines, ultimately benefiting patients awaiting innovative treatment options.
As per cell and gene therapy manufacturing industry trends, North America dominated the global industry in 2022. The region is home to a robust and well-established pharmaceutical and biotechnology industry.
Rise in prevalence of chronic diseases has amplified the demand for advanced treatment options, driving investment and research in cell and gene therapies. Additionally, the region's well-established healthcare infrastructure and regulatory framework provide a conducive environment for developing and commercializing manufacturing processes.
Collaborations between academia, research institutions, and pharmaceutical companies are fostering innovation and knowledge sharing, accelerating the translation of scientific discoveries into marketable therapies. Surge in funding from both public and private sectors further supports research and infrastructure development, enabling scalability and efficiency in manufacturing processes.
Technological advancements play a pivotal role, with automation, data analytics, and process optimization enhancing productivity and quality control. As a result, industry players are attracted to investing in North America to harness the benefits of cutting-edge technologies.
According to cell and gene therapy manufacturing market forecast, the industry in Asia Pacific is expected to grow at a rapid pace during the forecast period. This can be ascribed to expanding healthcare infrastructure and increasing research & development investment in the region.
Favorable regulatory policies and streamlined approval processes in countries such as China, Japan, and South Korea facilitate quicker market entry for cell and gene therapy products. Moreover, a large population base and rise in prevalence of chronic diseases in the region drive demand for advanced treatment options, boosting the market's expansion.
Collaborations between academic institutions, biotech companies, and government bodies enhance expertise and knowledge sharing, propelling innovation. Availability of skilled workforce and cost-effective manufacturing capabilities further attract global biopharmaceutical players to set up production facilities in Asia Pacific, solidifying the region's position at the forefront of cell and gene therapy manufacturing.
The global cell and gene therapy manufacturing market is fragmented, with the presence of large a number of players. Increase in investment in R&D and merger & acquisition are the key strategies adopted by leading companies.
Lonza Group Ltd., Thermo Fisher Scientific, Inc., Merck KGaA, Danaher Corporation, Bio-Techne Corporation, Sartorius AG (Sartorius Stedim Biotech S.A.), Bio-Rad Laboratories, Inc., Becton, Dickinson and Company, Fresenius Medical Care AG & Co. KGaA, MaxCyte, Getinge AB, and GE HealthCare.
Each of these players has been profiled in the cell and gene therapy manufacturing market research report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2022 | US$ 1.1 Bn |
| Forecast Value in 2031 | More than US$ 8.8 Bn |
| CAGR - 2022-2031 | 25.2% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2021 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 1.1 Bn in 2022
It is projected to reach more than US$ 8.8 Bn by 2031
The CAGR is anticipated to be 25.2% from 2023 to 2031
North America is expected to account for the largest share from 2023 to 2031
Lonza Group Ltd., Thermo Fisher Scientific, Inc., Merck KGaA, Danaher Corporation, Bio-Techne Corporation, Sartorius AG (Sartorius Stedim Biotech S.A.), Bio-Rad Laboratories, Inc., Becton, Dickinson and Company, Fresenius Medical Care AG & Co. KGaA, MaxCyte, Getinge AB, and GE HealthCare
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Cell and Gene Therapy Manufacturing Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, 2017-2031
5. Key Insights
5.1. Technological Advancements
5.2. Reimbursement Scenario by Region/Globally
5.3. Regulatory Scenario by Region/globally
5.4. Disease Prevalence & Incidence Rate Globally With Key Countries
5.5. COVID-19 Pandemic Impact on Industry (value chain and short/mid/long term impact)
6. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, by Therapy Type
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Therapy Type, 2017-2031
6.3.1. Cell Therapy
6.3.1.1. Allogeneic
6.3.1.1.1. Mesenchymal Stem Cells
6.3.1.1.2. T-cells
6.3.1.1.3. Induced Pluripotent Stem Cells
6.3.1.1.4. Natural Killer Cells
6.3.1.1.5. Hematopoietic Stem Cells
6.3.1.1.6. Others
6.3.1.2. Autologous
6.3.1.2.1. T-cells
6.3.1.2.2. Hematopoietic Stem Cells
6.3.1.2.3. Mesenchymal Stem Cells
6.3.1.2.4. Natural Killer Cells
6.3.1.2.5. Others
6.3.2. Gene Therapy
6.3.2.1. Viral Vector
6.3.2.1.1. Lentiviral Vector
6.3.2.1.2. Adeno-associated Virus Vectors
6.3.2.1.3. Other Viral Vectors
6.3.2.2. Non-viral Vector
6.3.2.2.1. Electroporation
6.3.2.2.2. Other Non-viral Vectors
6.4. Market Attractiveness Analysis, by Therapy Type
7. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, by Product Type
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Product Type, 2017-2031
7.3.1. Consumables
7.3.2. Equipment
7.3.2.1. Cell Processing Equipment
7.3.2.2. Single-use Equipment
7.3.2.3. Other Equipment
7.3.3. Software & Systems
7.4. Market Attractiveness Analysis, by Product Type
8. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, by Technology
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by Technology, 2017-2031
8.3.1. Cell Culture & Expansion
8.3.2. Viral Vector Production
8.3.3. Cell Sorting & Purification
8.3.4. Gene Editing
8.3.5. Others
8.4. Market Attractiveness Analysis, by Technology
9. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, by Indication
9.1. Introduction & Definition
9.2. Key Findings/Developments
9.3. Market Value Forecast, by Indication, 2017-2031
9.3.1. Oncology Disease
9.3.2. Cardiovascular Disease
9.3.3. Orthopedic Disease
9.3.4. Infectious Disease
9.3.5. Others
9.4. Market Attractiveness Analysis, by Indication
10. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, by End-user
10.1. Introduction & Definition
10.2. Key Findings/Developments
10.3. Market Value Forecast, by End-user, 2017-2031
10.3.1. Pharmaceutical & Biotechnology Companies
10.3.2. Contract Manufacturing Organizations (CMOs)
10.4. Market Attractiveness Analysis, by End-user
11. Cell and Gene Therapy Manufacturing Market Analysis and Forecast, by Region
11.1. Key Findings
11.2. Market Value Forecast, by Region, 2017-2031
11.2.1. North America
11.2.2. Europe
11.2.3. Asia Pacific
11.2.4. Latin America
11.2.5. Middle East & Africa
11.3. Market Attractiveness Analysis, by Region
12. North America Cell and Gene Therapy Manufacturing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Therapy Type, 2017-2031
12.2.1. Cell Therapy
12.2.1.1. Allogeneic
12.2.1.1.1. Mesenchymal Stem Cells
12.2.1.1.2. T-cells
12.2.1.1.3. Induced Pluripotent Stem Cells
12.2.1.1.4. Natural Killer Cells
12.2.1.1.5. Hematopoietic Stem Cells
12.2.1.1.6. Others
12.2.1.2. Autologous
12.2.1.2.1. T-cells
12.2.1.2.2. Hematopoietic Stem Cells
12.2.1.2.3. Mesenchymal Stem Cells
12.2.1.2.4. Natural Killer Cells
12.2.1.2.5. Others
12.2.2. Gene Therapy
12.2.2.1. Viral Vector
12.2.2.1.1. Lentiviral Vector
12.2.2.1.2. Adeno-associated Virus Vectors
12.2.2.1.3. Other Viral Vectors
12.2.2.2. Non-viral Vector
12.2.2.2.1. Electroporation
12.2.2.2.2. Other Non-viral Vectors
12.3. Market Value Forecast, by Product Type, 2017-2031
12.3.1. Consumables
12.3.2. Equipment
12.3.2.1. Cell Processing Equipment
12.3.2.2. Single-use Equipment
12.3.2.3. Other Equipment
12.3.3. Software & Systems
12.4. Market Value Forecast, by Technology, 2017-2031
12.4.1. Cell Culture & Expansion
12.4.2. Viral Vector Production
12.4.3. Cell Sorting & Purification
12.4.4. Gene Editing
12.4.5. Others
12.5. Market Value Forecast, by Indication, 2017-2031
12.5.1. Oncology Disease
12.5.2. Cardiovascular Disease
12.5.3. Orthopedic Disease
12.5.4. Infectious Disease
12.5.5. Others
12.6. Market Value Forecast, by End-user, 2017-2031
12.6.1. Pharmaceutical & Biotechnology Companies
12.6.2. Contract Manufacturing Organizations (CMOs)
12.7. Market Value Forecast, by Country, 2017-2031
12.7.1. U.S.
12.7.2. Canada
12.8. Market Attractiveness Analysis
12.8.1. By Therapy Type
12.8.2. By Product Type
12.8.3. By Technology
12.8.4. By Indication
12.8.5. By End-user
12.8.6. By Country
13. Europe Cell and Gene Therapy Manufacturing Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Therapy Type, 2017-2031
13.2.1. Cell Therapy
13.2.1.1. Allogeneic
13.2.1.1.1. Mesenchymal Stem Cells
13.2.1.1.2. T-cells
13.2.1.1.3. Induced Pluripotent Stem Cells
13.2.1.1.4. Natural Killer Cells
13.2.1.1.5. Hematopoietic Stem Cells
13.2.1.1.6. Others
13.2.1.2. Autologous
13.2.1.2.1. T-cells
13.2.1.2.2. Hematopoietic Stem Cells
13.2.1.2.3. Mesenchymal Stem Cells
13.2.1.2.4. Natural Killer Cells
13.2.1.2.5. Others
13.2.2. Gene Therapy
13.2.2.1. Viral Vector
13.2.2.1.1. Lentiviral Vector
13.2.2.1.2. Adeno-associated Virus Vectors
13.2.2.1.3. Other Viral Vectors
13.2.2.2. Non-viral Vector
13.2.2.2.1. Electroporation
13.2.2.2.2. Other Non-viral Vectors
13.3. Market Value Forecast, by Product Type, 2017-2031
13.3.1. Consumables
13.3.2. Equipment
13.3.2.1. Cell Processing Equipment
13.3.2.2. Single-use Equipment
13.3.2.3. Other Equipment
13.3.3. Software & Systems
13.4. Market Value Forecast, by Technology, 2017-2031
13.4.1. Cell Culture & Expansion
13.4.2. Viral Vector Production
13.4.3. Cell Sorting & Purification
13.4.4. Gene Editing
13.4.5. Others
13.5. Market Value Forecast, by Indication, 2017-2031
13.5.1. Oncology Disease
13.5.2. Cardiovascular Disease
13.5.3. Orthopedic Disease
13.5.4. Infectious Disease
13.5.5. Others
13.6. Market Value Forecast, by End-user, 2017-2031
13.6.1. Pharmaceutical & Biotechnology Companies
13.6.2. Contract Manufacturing Organizations (CMOs)
13.7. Market Value Forecast, by Country/Sub-region, 2017-2031
13.7.1. Germany
13.7.2. U.K.
13.7.3. France
13.7.4. Italy
13.7.5. Spain
13.7.6. Rest of Europe
13.8. Market Attractiveness Analysis
13.8.1. By Therapy Type
13.8.2. By Product Type
13.8.3. By Technology
13.8.4. By Indication
13.8.5. By End-user
13.8.6. By Country/Sub-region
14. Asia Pacific Cell and Gene Therapy Manufacturing Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Therapy Type, 2017-2031
14.2.1. Cell Therapy
14.2.1.1. Allogeneic
14.2.1.1.1. Mesenchymal Stem Cells
14.2.1.1.2. T-cells
14.2.1.1.3. Induced Pluripotent Stem Cells
14.2.1.1.4. Natural Killer Cells
14.2.1.1.5. Hematopoietic Stem Cells
14.2.1.1.6. Others
14.2.1.2. Autologous
14.2.1.2.1. T-cells
14.2.1.2.2. Hematopoietic Stem Cells
14.2.1.2.3. Mesenchymal Stem Cells
14.2.1.2.4. Natural Killer Cells
14.2.1.2.5. Others
14.2.2. Gene Therapy
14.2.2.1. Viral Vector
14.2.2.1.1. Lentiviral Vector
14.2.2.1.2. Adeno-associated Virus Vectors
14.2.2.1.3. Other Viral Vectors
14.2.2.2. Non-viral Vector
14.2.2.2.1. Electroporation
14.2.2.2.2. Other Non-viral Vectors
14.3. Market Value Forecast, by Product Type, 2017-2031
14.3.1. Consumables
14.3.2. Equipment
14.3.2.1. Cell Processing Equipment
14.3.2.2. Single-use Equipment
14.3.2.3. Other Equipment
14.3.3. Software & Systems
14.4. Market Value Forecast, by Technology, 2017-2031
14.4.1. Cell Culture & Expansion
14.4.2. Viral Vector Production
14.4.3. Cell Sorting & Purification
14.4.4. Gene Editing
14.4.5. Others
14.5. Market Value Forecast, by Indication, 2017-2031
14.5.1. Oncology Disease
14.5.2. Cardiovascular Disease
14.5.3. Orthopedic Disease
14.5.4. Infectious Disease
14.5.5. Others
14.6. Market Value Forecast, by End-user, 2017-2031
14.6.1. Pharmaceutical & Biotechnology Companies
14.6.2. Contract Manufacturing Organizations (CMOs)
14.7. Market Value Forecast, by Country/Sub-region, 2017-2031
14.7.1. China
14.7.2. Japan
14.7.3. India
14.7.4. Australia & New Zealand
14.7.5. Rest of Asia Pacific
14.8. Market Attractiveness Analysis
14.8.1. By Therapy Type
14.8.2. By Product Type
14.8.3. By Technology
14.8.4. By Indication
14.8.5. By End-user
14.8.6. By Country/Sub-region
15. Latin America Cell and Gene Therapy Manufacturing Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Therapy Type, 2017-2031
15.2.1. Cell Therapy
15.2.1.1. Allogeneic
15.2.1.1.1. Mesenchymal Stem Cells
15.2.1.1.2. T-cells
15.2.1.1.3. Induced Pluripotent Stem Cells
15.2.1.1.4. Natural Killer Cells
15.2.1.1.5. Hematopoietic Stem Cells
15.2.1.1.6. Others
15.2.1.2. Autologous
15.2.1.2.1. T-cells
15.2.1.2.2. Hematopoietic Stem Cells
15.2.1.2.3. Mesenchymal Stem Cells
15.2.1.2.4. Natural Killer Cells
15.2.1.2.5. Others
15.2.2. Gene Therapy
15.2.2.1. Viral Vector
15.2.2.1.1. Lentiviral Vector
15.2.2.1.2. Adeno-associated Virus Vectors
15.2.2.1.3. Other Viral Vectors
15.2.2.2. Non-viral Vector
15.2.2.2.1. Electroporation
15.2.2.2.2. Other Non-viral Vectors
15.3. Market Value Forecast, by Product Type, 2017-2031
15.3.1. Consumables
15.3.2. Equipment
15.3.2.1. Cell Processing Equipment
15.3.2.2. Single-use Equipment
15.3.2.3. Other Equipment
15.3.3. Software & Systems
15.4. Market Value Forecast, by Technology, 2017-2031
15.4.1. Cell Culture & Expansion
15.4.2. Viral Vector Production
15.4.3. Cell Sorting & Purification
15.4.4. Gene Editing
15.4.5. Others
15.5. Market Value Forecast, by Indication, 2017-2031
15.5.1. Oncology Disease
15.5.2. Cardiovascular Disease
15.5.3. Orthopedic Disease
15.5.4. Infectious Disease
15.5.5. Others
15.6. Market Value Forecast, by End-user, 2017-2031
15.6.1. Pharmaceutical & Biotechnology Companies
15.6.2. Contract Manufacturing Organizations (CMOs)
15.7. Market Value Forecast, by Country/Sub-region, 2017-2031
15.7.1. Brazil
15.7.2. Mexico
15.7.3. Rest of Latin America
15.8. Market Attractiveness Analysis
15.8.1. By Therapy Type
15.8.2. By Product Type
15.8.3. By Technology
15.8.4. By Indication
15.8.5. By End-user
15.8.6. By Country/Sub-region
16. Middle East & Africa Cell and Gene Therapy Manufacturing Market Analysis and Forecast
16.1. Introduction
16.1.1. Key Findings
16.2. Market Value Forecast, by Therapy Type, 2017-2031
16.2.1. Cell Therapy
16.2.1.1. Allogeneic
16.2.1.1.1. Mesenchymal Stem Cells
16.2.1.1.2. T-cells
16.2.1.1.3. Induced Pluripotent Stem Cells
16.2.1.1.4. Natural Killer Cells
16.2.1.1.5. Hematopoietic Stem Cells
16.2.1.1.6. Others
16.2.1.2. Autologous
16.2.1.2.1. T-cells
16.2.1.2.2. Hematopoietic Stem Cells
16.2.1.2.3. Mesenchymal Stem Cells
16.2.1.2.4. Natural Killer Cells
16.2.1.2.5. Others
16.2.2. Gene Therapy
16.2.2.1. Viral Vector
16.2.2.1.1. Lentiviral Vector
16.2.2.1.2. Adeno-associated Virus Vectors
16.2.2.1.3. Other Viral Vectors
16.2.2.2. Non-viral Vector
16.2.2.2.1. Electroporation
16.2.2.2.2. Other Non-viral Vectors
16.3. Market Value Forecast, by Product Type, 2017-2031
16.3.1. Consumables
16.3.2. Equipment
16.3.2.1. Cell Processing Equipment
16.3.2.2. Single-use Equipment
16.3.2.3. Other Equipment
16.3.3. Software & Systems
16.4. Market Value Forecast, by Technology, 2017-2031
16.4.1. Cell Culture & Expansion
16.4.2. Viral Vector Production
16.4.3. Cell Sorting & Purification
16.4.4. Gene Editing
16.4.5. Others
16.5. Market Value Forecast, by Indication, 2017-2031
16.5.1. Oncology Disease
16.5.2. Cardiovascular Disease
16.5.3. Orthopedic Disease
16.5.4. Infectious Disease
16.5.5. Others
16.6. Market Value Forecast, by End-user, 2017-2031
16.6.1. Pharmaceutical & Biotechnology Companies
16.6.2. Contract Manufacturing Organizations (CMOs)
16.7. Market Value Forecast, by Country/Sub-region, 2017-2031
16.7.1. GCC Countries
16.7.2. South Africa
16.7.3. Rest of Middle East & Africa
16.8. Market Attractiveness Analysis
16.8.1. By Therapy Type
16.8.2. By Product Type
16.8.3. By Technology
16.8.4. By Indication
16.8.5. By End-user
16.8.6. By Country/Sub-region
17. Competition Landscape
17.1. Market Player - Competition Matrix (By Tier and Size of Companies)
17.2. Market Share Analysis, by Company (2022)
17.3. Company Profiles
17.3.1. Becton, Dickinson and Company
17.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.1.2. Product Portfolio
17.3.1.3. Financial Overview
17.3.1.4. SWOT Analysis
17.3.1.5. Strategic Overview
17.3.2. Bio-Rad Laboratories, Inc.
17.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.2.2. Product Portfolio
17.3.2.3. Financial Overview
17.3.2.4. SWOT Analysis
17.3.2.5. Strategic Overview
17.3.3. Bio-Techne Corporation
17.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.3.2. Product Portfolio
17.3.3.3. Financial Overview
17.3.3.4. SWOT Analysis
17.3.3.5. Strategic Overview
17.3.4. Danaher Corporation
17.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.4.2. Product Portfolio
17.3.4.3. Financial Overview
17.3.4.4. SWOT Analysis
17.3.4.5. Strategic Overview
17.3.5. General Electric Company (GE Healthcare)
17.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.5.2. Product Portfolio
17.3.5.3. Financial Overview
17.3.5.4. SWOT Analysis
17.3.5.5. Strategic Overview
17.3.6. Getinge AB
17.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.6.2. Product Portfolio
17.3.6.3. Financial Overview
17.3.6.4. SWOT Analysis
17.3.6.5. Strategic Overview
17.3.7. Lonza Group Ltd.
17.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.7.2. Product Portfolio
17.3.7.3. Financial Overview
17.3.7.4. SWOT Analysis
17.3.7.5. Strategic Overview
17.3.8. Merck KGaA
17.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.8.2. Product Portfolio
17.3.8.3. Financial Overview
17.3.8.4. SWOT Analysis
17.3.8.5. Strategic Overview
17.3.9. Sartorius AG (Sartorius Stedim Biotech S.A.)
17.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.9.2. Product Portfolio
17.3.9.3. Financial Overview
17.3.9.4. SWOT Analysis
17.3.9.5. Strategic Overview
17.3.10. Thermo Fisher Scientific Inc.
17.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.10.2. Product Portfolio
17.3.10.3. Financial Overview
17.3.10.4. SWOT Analysis
17.3.10.5. Strategic Overview
17.3.11. MaxCyte
17.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.11.2. Product Portfolio
17.3.11.3. Financial Overview
17.3.11.4. SWOT Analysis
17.3.11.5. Strategic Overview
17.3.12. Fresenius Medical Care AG & Co. KGaA
17.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.12.2. Product Portfolio
17.3.12.3. Financial Overview
17.3.12.4. SWOT Analysis
17.3.12.5. Strategic Overview
List of Tables
Table 01: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Therapy Type, 2017-2031
Table 02: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Cell Therapy, 2017-2031
Table 03: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Allogeneic, 2017-2031
Table 04: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Autologous, 2017-2031
Table 05: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 06: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 07: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Viral Vector, 2017-2031
Table 08: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Non-viral Vector, 2017-2031
Table 09: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 10: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Technology, 2017‒2031
Table 11: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Indication, 2017-2031
Table 12: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 13: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Region, 2017-2031
Table 14: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Country, 2017-2031
Table 15: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Therapy Type, 2017-2031
Table 16: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Cell Therapy, 2017-2031
Table 17: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Allogeneic, 2017-2031
Table 18: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Autologous, 2017-2031
Table 19: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 20: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Viral Vector, 2017-2031
Table 21: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Non-viral Vector, 2017-2031
Table 22: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 23: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Equipment, 2017-2031
Table 24: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Technology, 2017‒2031
Table 25: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Indication, 2017-2031
Table 26: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 27: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 28: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Therapy Type, 2017-2031
Table 29: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Cell Therapy, 2017-2031
Table 30: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Allogeneic, 2017-2031
Table 31: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Autologous, 2017-2031
Table 32: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 33: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Viral Vector, 2017-2031
Table 34: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Non-viral Vector, 2017-2031
Table 35: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 36: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Equipment, 2017-2031
Table 37: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Technology, 2017‒2031
Table 38: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Indication, 2017-2031
Table 39: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 40: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 41: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Therapy Type, 2017-2031
Table 42: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Cell Therapy, 2017-2031
Table 43: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Allogeneic, 2017-2031
Table 44: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Autologous, 2017-2031
Table 45: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 46: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Viral Vector, 2017-2031
Table 47: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Non-viral Vector, 2017-2031
Table 48: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 49: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Equipment, 2017-2031
Table 50: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Technology, 2017‒2031
Table 51: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Indication, 2017-2031
Table 52: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 53: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 54: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Therapy Type, 2017-2031
Table 55: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Cell Therapy, 2017-2031
Table 56: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Allogeneic, 2017-2031
Table 57: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Autologous, 2017-2031
Table 58: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 59: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Viral Vector, 2017-2031
Table 60: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Non-viral Vector, 2017-2031
Table 61: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 62: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Equipment, 2017-2031
Table 63: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Technology, 2017‒2031
Table 64: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Indication, 2017-2031
Table 65: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 66: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 67: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Therapy Type, 2017-2031
Table 68: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Cell Therapy, 2017-2031
Table 69: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Allogeneic, 2017-2031
Table 70: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Autologous, 2017-2031
Table 71: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Gene Therapy, 2017-2031
Table 72: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Viral Vector, 2017-2031
Table 73: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Non-viral Vector, 2017-2031
Table 74: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 75: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Equipment, 2017-2031
Table 76: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Technology, 2017‒2031
Table 77: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by Indication, 2017-2031
Table 78: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, by End-user, 2017-2031
List of Figures
Figure 01: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, 2017-2031
Figure 02: Global Cell and Gene Therapy Manufacturing Market Value Share, by Therapy Type, 2022
Figure 03: Global Cell and Gene Therapy Manufacturing Market Value Share, by Product Type, 2022
Figure 04: Global Cell and Gene Therapy Manufacturing Market Value Share, by Indication, 2022
Figure 05: Global Cell and Gene Therapy Manufacturing Market Value Share, by Technology, 2022
Figure 06: Global Cell and Gene Therapy Manufacturing Market Value Share, by End-user, 2022
Figure 07: Global Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Therapy Type, 2022 and 2031
Figure 08: Global Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Therapy Type, 2023-2031
Figure 09: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Cell Therapy, 2017-2031
Figure 10: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Gene Therapy, 2017-2031
Figure 11: Global Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Product Type, 2022 and 2031
Figure 12: Global Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 13: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Consumables, 2017-2031
Figure 14: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Viral Vector Production, 2017‒2031
Figure 15: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Others, 2017‒2031
Figure 16: Global Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 17: Global Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Technology 2023-2031
Figure 18: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Cell Culture & Expansion, 2017‒2031
Figure 19: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Viral Vector Production, 2017‒2031
Figure 20: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Irreversible Cell Sorting & Purification, 2017‒2031
Figure 21: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Gene Editing, 2017‒2031
Figure 22: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Others, 2017‒2031
Figure 23: Global Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Indication, 2022 and 2031
Figure 24: Global Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, Indication, 2023-2031
Figure 25: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Oncology, 2017-2031
Figure 26: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Cardiovascular Disorders, 2017-2031
Figure 27: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Orthopedic Disease, 2017-2031
Figure 28: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Infectious Disease, 2017-2031
Figure 29: Global Cell and Gene Therapy Manufacturing Market Value (US$ Mn), by Others, 2017‒2031
Figure 30: Global Cell and Gene Therapy Manufacturing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 31: Global Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 32: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Pharmaceutical & Biotechnology Companies, 2017-2031
Figure 33: Global Cell and Gene Therapy Manufacturing Market Revenue (US$ Mn), by Contract Manufacturing Organizations (CMOs), 2017-2031
Figure 34: Global Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Region, 2022 and 2031
Figure 35: Global Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Region, 2023-2031
Figure 36: North America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, 2017-2031
Figure 37: North America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Country, 2022 and 2031
Figure 38: North America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Country, 2023-2031
Figure 39: North America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Therapy Type, 2022 and 2031
Figure 40: North America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Therapy Type, 2023-2031
Figure 41: North America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Product Type, 2022 and 2031
Figure 42: North America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 43: North America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 44: North America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Technology 2023-2031
Figure 45: North America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Indication, 2022 and 2031
Figure 46: North America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, Indication, 2023-2031
Figure 47: North America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 48: North America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 49: Europe Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, 2017-2031
Figure 50: Europe Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 51: Europe Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 52: Europe Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Therapy Type, 2022 and 2031
Figure 53: Europe Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Therapy Type, 2023-2031
Figure 54: Europe Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Product Type, 2022 and 2031
Figure 55: Europe Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 56: Europe Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 57: Europe Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Technology 2023-2031
Figure 58: Europe Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Indication, 2022 and 2031
Figure 59: Europe Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, Indication, 2023-2031
Figure 60: Europe Cell and Gene Therapy Manufacturing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 61: Europe Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 62: Asia Pacific Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, 2017-2031
Figure 63: Asia Pacific Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 64: Asia Pacific Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 65: Asia Pacific Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Therapy Type, 2022 and 2031
Figure 66: Asia Pacific Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Therapy Type, 2023-2031
Figure 67: Asia Pacific Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Product Type, 2022 and 2031
Figure 68: Asia Pacific Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 69: Asia Pacific Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 70: Asia Pacific Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Technology 2023-2031
Figure 71: Asia Pacific Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Indication, 2022 and 2031
Figure 72: Asia Pacific Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, Indication, 2023-2031
Figure 73: Asia Pacific Cell and Gene Therapy Manufacturing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 74: Asia Pacific Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 75: Latin America Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, 2017-2031
Figure 76: Latin America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 77: Latin America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 78: Latin America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Therapy Type, 2022 and 2031
Figure 79: Latin America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Therapy Type, 2023-2031
Figure 80: Latin America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Product Type, 2022 and 2031
Figure 81: Latin America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 82: Latin America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 83: Latin America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Technology 2023-2031
Figure 84: Latin America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Indication, 2022 and 2031
Figure 85: Latin America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, Indication, 2023-2031
Figure 86: Latin America Cell and Gene Therapy Manufacturing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 87: Latin America Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by End-user, 2023-2031
Figure 88: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value (US$ Mn) Forecast, 2017-2031
Figure 89: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 90: Middle East & Africa Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 91: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Therapy Type, 2022 and 2031
Figure 92: Middle East & Africa Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Therapy Type, 2023-2031
Figure 93: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Product Type, 2022 and 2031
Figure 94: Middle East & Africa Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 95: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Technology, 2022 and 2031
Figure 96: Middle East & Africa Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by Technology 2023-2031
Figure 97: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value Share Analysis, by Indication, 2022 and 2031
Figure 98: Middle East & Africa Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, Indication, 2023-2031
Figure 99: Middle East & Africa Cell and Gene Therapy Manufacturing Market Value Share Analysis, by End-user, 2022 and 2031
Figure 100: Middle East & Africa Cell and Gene Therapy Manufacturing Market Attractiveness Analysis, by End-user, 2023-2031