Reports
Reports
Global Pharmaceutical Continuous Manufacturing Technology Market: Snapshot
In pharmaceutical continuous manufacturing technology, active ingredients are produced in compact, closed units, leveraging automation. Hence, it requires fewer manual interventions. Production steps carried out sequentially in a classic batch process are combined in a continuous process. All these help to bring about continued utilization of production capacity. This in turn serves to reduce fluctuations in production, improve yields, and lower costs of operation and equipment. On account of such advantages, the method is seeing fast adoption.
Other advantages of pharmaceutical continuous manufacturing technology is the superior development speeds, higher process safety when employing hazardous chemistries, and the opportunity to perform reactions that cannot be run under batch processing.
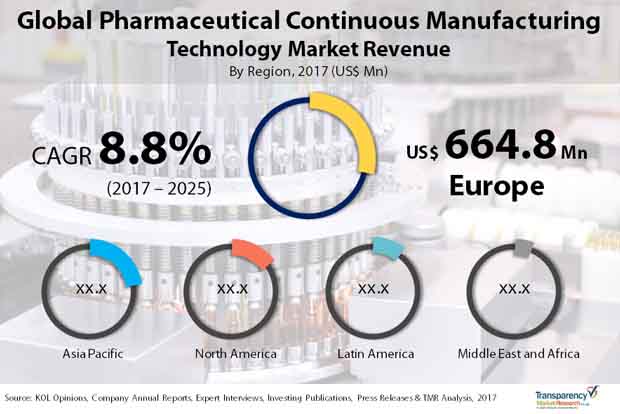
A study by Transparency Market Research forecasts the global pharmaceutical continuous manufacturing technology market to expand at a healthy CAGR of 8.8% from 2017 to 2025. Expanding at this pace, the market which was worth US$1.74 bn in 2016 is expected to attain a value of US$3.693 bn in 2025.
Contract Manufacturing Organizations in Asia Pacific to Drive Market
Some of the key application segments of the global pharmaceutical continuous manufacturing technology market are biologics, dry powders, active pharmaceutical ingredients, etc. Of them, the segment of biologics accounts for a dominant position in the market. It held a share of about 35.3% in the global market in 2016. It was trailed by active pharmaceutical ingredient.
Application-wise, the global pharmaceutical continuous manufacturing technology market can be divided into contract manufacturing organizations, pharmaceutical companies, etc. Currently, pharmaceutical companies lead the market with maximum demand and are trailed by contract manufacturing organizations. In the near future, the contract manufacturing organizations in Asia Pacific region are predicted to fuel market growth majorly. Along with increasing awareness about the technology, various initiatives by FDA are also egging manufacturers to shift to continuous manufacturing over batch manufacturing process.
Demand for Better Technology Propels Europe Market to Fore
From a geographical standpoint, currently Europe dominates the global pharmaceutical continuous manufacturing technology market. Strong demand for advanced technology from pharmaceutical companies and presence of many contract manufacturing entities has majorly fuelled the market in the region. The growth in Europe is primarily powered by the U.K. and Germany. The region’s leading share in 2016 came to around 35%. The market in the region is also expected to outshine others in terms of growth by registering a CAGR of 9.6% in the forecast period. Expanding at this pace, it is predicted to clock a revenue of US$1.38 bn by 2025. This is mainly on account of the early availability of advanced technologies and greater number of technology providers across the region.
Asia Pacific trails Europe vis-à-vis market share. In 2016, the region held a share of about 34% in the global pharmaceutical continuous manufacturing market. The growth in Asia Pacific has been mainly driven by China and India, which are home to numerous contract manufacturing organizations. China is considered a highly promising market because of increasing acceptability of technologically advanced continuous manufacturing and growing awareness about the advantages of the technology.
North America comes in the third position in terms of market size. Initiatives by FDA to switch from current batch process to continuous manufacturing technology drives interest among the pharmaceutical companies.
Some of the prominent participants in the global pharmaceutical continuous manufacturing technology market are Siemens AG, GEA group, Continuus Pharmaceutical, S K Biotek ltd, Korsch AG, Scott Equipment Company, Corning Life Sciences, and Chemtrix.
Heavy Advantages of Continuous Process Manufacturing to Boost Pharmaceutical Continuous Manufacturing Market
Continuous manufacturing is a high level manufacturing approach with the possibility to improve quality and consistency of drugs with lower cost. This creation line is worked in a continuous stream, with start to finish incorporation of manufacturing measures. The pharmaceutical continuous manufacturing market is required to observe critical development during the conjecture time frame, because of innovative progressions in continuous manufacturing frameworks and backing by the administrative experts for selection of continuous manufacturing frameworks. Nonetheless, greater expense of pharmaceutical continuous manufacturing frameworks obstructs the pharmaceutical continuous manufacturing market development.
Asia-Pacific is expected to rise remarkably in the coming years because of expansion in mindfulness about cutting edge pharmaceutical continuous manufacturing frameworks, rise number of pharmaceutical organizations, and expansion sought after for continuous manufacturing frameworks. Notwithstanding, greater expense of pharmaceutical continuous manufacturing frameworks may hamper the market development in Asia-Pacific.
Manufacturing innovation gives a superior method of manufacturing drug items that save time, improve quality, lessen required creation and use of creation limit in continuous manufacturing are excepted to be significant drivers of the worldwide pharmaceutical continuous manufacturing market. In any case, the high execution cost of computerized units for continuous manufacturing is restriction in market development. Also, the business the scholarly world coordinated efforts, development in nonexclusive manufacturing are making beneficial and joint endeavors among the main entertainer are the significant chances in the worldwide market.
Continuous manufacturing measure can accelerate the manufacturing cycle and improve wellbeing while at the same time utilizing perilous synthetic and lead hazardous interaction. It improves proficiency of the pharmaceutical manufacturing are boosting reception of the pharmaceutical continuous manufacturing measure across various organizations which is driving development of the worldwide pharmaceutical continuous manufacturing market.
Regardless of these development prospects, the absence of specialized and administrative clearness are restricting selection of the pharmaceutical continuous manufacturing measure which is hampering development of the worldwide pharmaceutical continuous manufacturing market. Furthermore, continuous cycle is required to have development prospect attributable to the developing of blend of complex innovations prompting precise observing, computerization, better gear, and programming.
1. Preface
1.1. Report Scope and Market Segmentation
1.2. Research Highlights
2. Assumptions and Research Methodology
2.1. Assumptions
2.2. Acronyms Used
2.3. Research Methodology
3. Executive Summary
3.1. Global Pharmaceutical Continuous Manufacturing Technology Market Snapshot
4. Market Overview
4.1. Product Overview
4.2. Market Opportunity Map
4.3. Key Industry Events
4.4. Global Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, 2015–2025
4.5. Global Pharmaceutical Continuous Manufacturing Technology Market Outlook
4.6. Porter’s Five Forces Analysis
5. Market Dynamics
5.1. Drivers and Restraints Snapshot Analysis
5.2. Drivers
5.2.1. Increased inefficiency in the manufacturing process
5.2.2. Safer production
5.2.3. Advantages over the batch manufacturing
5.2.4. Increased FDA initiatives
5.3. Restraints
5.3.1. Regulatory challenges
5.3.2. Difficulties in adoption
5.4. Opportunities
5.4.1. Improving Pharma Infrastructure
5.5. Global API Manufacturing Technology Suppliers Market Share Analysis, by Company, 2016 (estimated)
6. Global Pharmaceutical Continuous Manufacturing Technology Market, by Application
6.1. Introduction
6.2. Key Findings
6.3. Global Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application
6.4. Global Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Application
6.5. Global Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user
6.6. Global Pharmaceutical Continuous Manufacturing Technology Market Analysis, by Application
6.7. Global Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application
6.8. Key Trends
7. Global Pharmaceutical Continuous Manufacturing Technology Market Analysis, by End-user
7.1. Introduction
7.2. Key Findings
7.3. Global Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user
7.4. Global Pharmaceutical Continuous Manufacturing Technology Market Forecast, by End-user
7.5. Global Pharmaceutical Continuous Manufacturing Technology Market Analysis, by End-user
7.6. Global Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user
7.7. Key Trends
8. Global Pharmaceutical Continuous Manufacturing Technology Market Analysis, by Region
8.1. Global Market Scenario, by Country
8.2. Global Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Region
8.3. Global Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Region, 2015–2025
8.4. Global Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Region
9. North America Pharmaceutical Continuous Manufacturing Technology Market Analysis
9.1. Key Findings
9.2. North America Pharmaceutical Continuous Manufacturing Technology Market Overview
9.3. North America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country
9.4. North America Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Country
9.5. North America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application
9.6. North America Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Application
9.7. North America Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user
9.8. North America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user
9.9. North America Pharmaceutical Continuous Manufacturing Technology Market Forecast, by End-user
9.10. North America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis
9.11. Key Trends
10. Europe Pharmaceutical Continuous Manufacturing Technology Market Analysis
10.1. Key Findings
10.2. Europe Pharmaceutical Continuous Manufacturing Technology Market Overview
10.3. Europe Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country
10.4. Europe Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Country
10.5. Europe Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application
10.6. Europe Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Application
10.7. Europe Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user
10.8. Europe Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user
10.9. Europe Pharmaceutical Continuous Manufacturing Technology Market Forecast, by End-user
10.10. Europe Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis
10.11. Key Trends
11. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Analysis
11.1. Key Findings
11.2. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Overview
11.3. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country
11.4. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Country
11.5. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application
11.6. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Application
11.7. Asia Pacific Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user
11.8. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user
11.9. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Forecast, by End-user
11.10. Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis
11.11. Key Trends
12. Latin America Pharmaceutical Continuous Manufacturing Technology Market Analysis
12.1. Key Findings
12.2. Latin America Pharmaceutical Continuous Manufacturing Technology Market Overview
12.3. Latin America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country
12.4. Latin America Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Country
12.5. Latin America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application
12.6. Latin America Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Application
12.7. Latin America Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user
12.8. Latin America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user
12.9. Latin America Pharmaceutical Continuous Manufacturing Technology Market Forecast, by End-user
12.10. Latin America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis
13. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Analysis
13.1. Key Findings
13.2. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Overview
13.3. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country
13.4. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Country
13.5. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application
13.6. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Forecast, by Application
13.7. Middle East & Africa Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user
13.8. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user
13.9. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Forecast, by End-user
13.10. Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis
14. Competition Landscape
14.1. Competition Matrix
14.2. Company Profiles
14.2.1. Siemens AG
14.2.1.1. Company Details
14.2.1.2. Business Overview
14.2.1.3. Financial Overview
14.2.1.4. Strategic Overview
14.2.1.5. SWOT Analysis
14.2.2. GEA Group
14.2.2.1. Company Details
14.2.2.2. Business Overview
14.2.2.3. Financial Overview
14.2.2.4. Strategic Overview
14.2.2.5. SWOT Analysis
14.2.3. SK biotek Co., Ltd.
14.2.3.1. Company Details
14.2.3.2. Business Overview
14.2.3.3. Financial Overview
14.2.3.4. Strategic Overview
14.2.3.5. SWOT Analysis
14.2.4. CONTINUUS Pharmaceuticals
14.2.4.1. Company Details
14.2.4.2. Business Overview
14.2.4.3. Financial Overview
14.2.4.4. Strategic Overview
14.2.4.5. SWOT Analysis
14.2.5. Gebrüder Lödige Maschinenbau GmbH
14.2.5.1. Company Details
14.2.5.2. Business Overview
14.2.5.3. Financial Overview
14.2.5.4. Strategic Overview
14.2.5.5. SWOT Analysis
14.2.6. Glatt GmbH
14.2.6.1. Company Details
14.2.6.2. Business Overview
14.2.6.3. Financial Overview
14.2.6.4. Strategic Overview
14.2.6.5. SWOT Analysis
14.2.7. KORSCH AG
14.2.7.1. Company Details
14.2.7.2. Business Overview
14.2.7.3. Financial Overview
14.2.7.4. Strategic Overview
14.2.7.5. SWOT Analysis
14.2.8. Scott Equipment Company
14.2.8.1. Company Details
14.2.8.2. Business Overview
14.2.8.3. Financial Overview
14.2.8.4. Strategic Overview
14.2.8.5. SWOT Analysis
14.2.9. Thermo Fisher Scientific, Inc.
14.2.9.1. Company Details
14.2.9.2. Business Overview
14.2.9.3. Financial Overview
14.2.9.4. Strategic Overview
14.2.9.5. SWOT Analysis
14.2.10. L.B. Bohle Maschinen + Verfahren Gmbh
14.2.10.1. Company Details
14.2.10.2. Business Overview
14.2.10.3. Financial Overview
14.2.10.4. Strategic Overview
14.2.10.5. SWOT Analysis
14.2.11. Munson Machinery Company
14.2.11.1. Company Details
14.2.11.2. Business Overview
14.2.11.3. Financial Overview
14.2.11.4. Strategic Overview
14.2.11.5. SWOT Analysis
List of Tables
TABLE 1 Revenue Share by Application
TABLE 2 Revenue Share by End-user
TABLE 3 Global Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Application, 2015–2025
TABLE 4 Global Pharmaceutical API Continuous Manufacturing Technology (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 5 Global Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 6 Global Pharmaceutical Continuous Manufacturing Technology Market Revenue (US$ Mn) Forecast, by Region, 2015–2025
TABLE 7 North America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Country, 2015–2025
TABLE 8 North America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Application, 2015–2025
TABLE 9 North America Pharmaceutical API Continuous Manufacturing Technology (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 10 North America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 11 Europe Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Country, 2015–2025
TABLE 12 Europe Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Application, 2015–2025
TABLE 13 Europe Pharmaceutical API Continuous Manufacturing Technology (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 14 Europe Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 15 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Country, 2015–2025
TABLE 16 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Application, 2015–2025
TABLE 17 Asia Pacific Pharmaceutical API Continuous Manufacturing Technology (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 18 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 19 Latin America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Country, 2015–2025
TABLE 20 Latin America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Application, 2015–2025
TABLE 21 Latin America Pharmaceutical API Continuous Manufacturing Technology Forecast, by End-user, 2015–2025
TABLE 22 Latin America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 23 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Country, 2015–2025
TABLE 24 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by Application, 2015–2025
TABLE 25 Middle East & Africa Pharmaceutical API Continuous Manufacturing Technology (US$ Mn) Forecast, by End-user, 2015–2025
TABLE 26 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, by End-user, 2015–2025
List of Figures
FIG. 1 Global Market Size (US$ Mn), by Application, 2016
FIG. 2 Global Market Size (US$ Mn), by End-users, 2016
FIG. 3 Key Trends in Continuous Manufacturing Technology
FIG. 4 Global Market share, by Region, 2016
FIG. 5 Key Industry Developments
FIG. 6 Global Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) Forecast, 2015–2025
FIG. 7 Market Value Share, by End-user (2016)
FIG. 8 Market Value Share, by Application (2016)
FIG. 9 Market Value Share, by Region (2016)
FIG. 10 Addressable Pharmaceutical Manufacturing Market
FIG. 11 Global API Manufacturing Technology Suppliers Market Share Analysis, by Company, 2016
FIG. 12 Global Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application, 2016 and 2025
FIG. 13 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth, by Active Pharmaceutical Ingredients (API), 2015–2025
FIG. 14 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth, by Biologics, 2015–2025
FIG. 15 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth, by Dry Powder, 2015–2025
FIG. 16 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth, by Others, 2015–2025
FIG. 17 Global Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application, 2015–2025
FIG. 18 Global Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user, 2016 and 2025
FIG. 19 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, by Pharmaceutical Companies, 2015–2025
FIG. 20 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, by Contract Manufacturing Organizations, 2015–2025
FIG. 21 Global Pharmaceutical Continuous Manufacturing Technology Market Value (US$ Mn) and Y-o-Y Growth (%) Forecast, by Others, 2015–2025
FIG. 22 Global Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user,2017–2025
FIG. 23 Global Market Scenario, by country
FIG. 24 Global Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Region, 2016 and 2025
FIG. 25 Global Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Region, 2017–2025
FIG. 26 North America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) and Y-o-Y Growth (%) Forecast, 2015–2025
FIG. 27 North America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Country, 2017–2025
FIG. 28 North America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country, 2016 and 2025
FIG. 29 North America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application, 2016 and 2025
FIG. 30 North America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user, 2016 and 2025
FIG. 31 North America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application, 2017–2025
FIG. 32 North America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user, 2017–2025
FIG. 33 Europe Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) and Y-o-Y Growth (%) Forecast, 2015–2025
FIG. 34 Europe Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Country, 2017–2025
FIG. 35 Europe Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country, 2016 and 2025
FIG. 36 Europe Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application, 2016 and 2025
FIG. 37 Europe Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user, 2016 and 2025
FIG. 38 Europe Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application, 2015–2025
FIG. 39 Europe Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user, 2015–2025
FIG. 40 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) and Y-o-Y Growth (%) Forecast, 2015–2025
FIG. 41 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Country, 2017–2025
FIG. 42 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country, 2016 and 2025
FIG. 43 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application, 2016 and 2025
FIG. 44 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user, 2016 and 2025
FIG. 45 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application, 2015–2025
FIG. 46 Asia Pacific Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user, 2015–2025
FIG. 47 Latin America Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) and Y-o-Y Growth (%) Forecast, 2015–2025
FIG. 48 Latin America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Country, 2017–2025
FIG. 49 Latin America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country, 2016 and 2025
FIG. 50 Latin America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application, 2016 and 2025
FIG. 51 Latin America Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user, 2016 and 2025
FIG. 52 Latin America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application, 2017–2025
FIG. 53 Latin America Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user, 2017–2025
FIG. 54 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Size (US$ Mn) and Y-o-Y Growth (%) Forecast, 2015–2025
FIG. 55 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Country, 2017–2025
FIG. 56 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Country, 2016 and 2025
FIG. 57 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by Application, 2016 and 2025
FIG. 58 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Value Share Analysis, by End-user, 2016 and 2025
FIG. 59 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by Application, 2017–2025
FIG. 60 Middle East & Africa Pharmaceutical Continuous Manufacturing Technology Market Attractiveness Analysis, by End-user, 2017–2025
FIG. 61 Siemens AG Breakdown of Net Sales, by Business Segment, 2016
FIG. 62 Siemens AG Total Revenue (US$ Bn) and Y-o-Y Growth (%), 2014–2016
FIG. 63 GEA Breakdown of Net Sales, by Business Segment, 2016
FIG. 64 GEA Revenue for (US$ Bn) and Y-o-Y Growth (%), 2014–2016
FIG. 65 SK biotek co. ltd Revenue (US$ Mn) and Y-o-Y Growth (%), 2015–2016
FIG. 66 Thermo Fischer Scientific, Inc Breakdown of Net Sales, by Region, 2016
FIG. 67 Thermo Fischer Scientific, Inc Revenue (US$ Bn) and Y-o-Y Growth (%), 2013–2016