Analyst Viewpoint
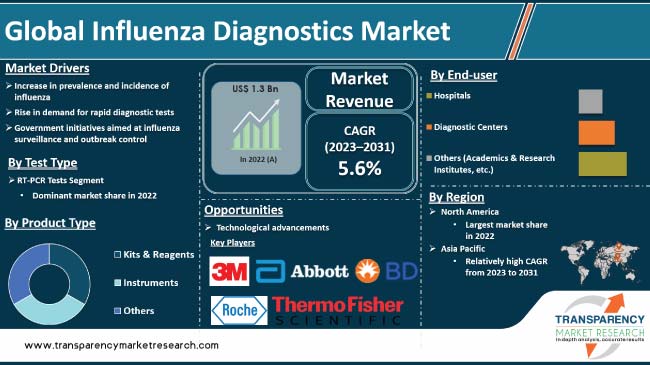
Increase in prevalence and incidence of influenza is driving the global influenza diagnostics market. Persistent threat of seasonal and pandemic influenza outbreaks have been witnessed in the past few years. The global impact of influenza on public health has prompted substantial investments in research & development. Government initiatives aimed at influenza surveillance and outbreak control are the major factors propelling market development. Furthermore, rise in demand for rapid diagnostic tests is expected to bolster the global influenza diagnostics industry size during the forecast period.
Technological advancements and introduction of innovative diagnostic tools offer lucrative opportunities to market players. Manufacturers are focusing on developing user-friendly, portable, and cost-effective diagnostic devices in order to increase market share.
Influenza, commonly known as the flu, is a highly contagious respiratory infection caused by influenza viruses. The impact of influenza on global public health underscores the importance of effective diagnostics for timely and accurate identification of the virus.
Influenza viruses belong to the Orthomyxoviridae family and are classified into types A, B, C, and D. Among these, influenza A and B are the predominant types responsible for seasonal outbreaks and pandemics.
Accurate and rapid influenza diagnostics play a crucial role in patient management, infection control, and public health surveillance. Various diagnostic methods are employed, ranging from traditional laboratory techniques such as viral culture and serological assays to more modern molecular techniques such as polymerase chain reaction (PCR) and rapid antigen tests.
Molecular methods offer high sensitivity and specificity, enabling the detection of viral RNA or DNA within hours, whereas rapid antigen tests provide quick results but may have lower sensitivity.
In the past few years, the global landscape has witnessed a notable surge in the occurrence of influenza, a contagious respiratory infection caused by influenza viruses. This upswing is multifactorial, influenced by various environmental, demographic, and virological factors. High transmission rates, compounded by the evolving nature of influenza viruses, underscore the pressing need for accurate and timely diagnostics. This in turn is fueling the global influenza diagnostics market.
Surge in prevalence of influenza can be ascribed to several factors, including increased globalization, urbanization, and population density. As individuals interact more frequently and over longer distances, the transmission of influenza viruses becomes more efficient.
Rapid urbanization has led to densely populated areas, creating environments conducive to rapid viral spread. These demographic shifts contribute significantly to the increasing burden of influenza on a global scale.
According to the World Health Organization, globally, there are around one billion cases of seasonal influenza annually, with 3 million to 5 million severe cases and 290,000 to 650,000 respiratory deaths. Thus, high disease burden fuels demand for efficient and accurate diagnostic tools.
The U.S. Centers for Disease Control and Prevention (CDC) reported early increase in influenza activity during the 2022–2023 season, particularly among children and adolescents. Hospitalization rates were also higher than in previous years.
The dynamic nature of influenza viruses, characterized by frequent mutations and antigenic drift, further exacerbates the challenge. This continual evolution enables the virus to evade immune responses, rendering previously exposed populations susceptible to reinfection. Consequently, demand for robust diagnostic tools becomes imperative for effective disease management and public health interventions.
In the realm of healthcare, the escalating prevalence of influenza is directly correlated with the rise in incidence of severe respiratory complications, hospitalizations, and mortality. This places a substantial burden on healthcare systems globally, necessitating swift and accurate diagnostic solutions to streamline patient management and implement appropriate preventive measures. This escalating prevalence and incidence of influenza is fueling the global influenza diagnostics market growth.
Surge in demand for Rapid Diagnostic Tests (RDTs) has emerged as a major factor propelling the global influenza diagnostics market demand. The ascendancy of RDTs as a primary diagnostic tool signifies a paradigm shift in influenza diagnostics, redefining the landscape with its inherent advantages. This escalating demand is underpinned by the urgent need for swift and accurate diagnostic outcomes, reflecting a discernible departure from conventional methodologies.
The prominence of RDTs can be ascribed to expeditious results, offering a real-time assessment of influenza infection status. This immediacy is imperative for timely clinical decision-making and initiation of appropriate therapeutic interventions.
Fast-paced lifestyles and the constant need for rapid responses in healthcare settings have increased preference for RDTs, positioning them as indispensable tools in influenza diagnostics.
Moreover, rise in demand for RDTs is intricately linked to their user-friendly nature, which facilitates decentralized testing. The accessibility of RDTs outside traditional laboratory settings ensures that diagnostic capabilities are extended to diverse healthcare settings, including remote and resource-constrained areas. This decentralization is pivotal in enhancing diagnostic reach and efficacy, particularly during influenza outbreaks or pandemics.
Cost-effectiveness of RDTs has further fueled adoption, aligning with the economic considerations of healthcare providers and patients alike. The overall reduction in turnaround time and the concomitant economic benefits associated with rapid diagnostics amplify the attractiveness of RDTs in the influenza diagnostics landscape. Thus, increase in demand for rapid diagnostic tests is likely to bolster the global influenza diagnostics market value.
In terms of test type, the RT-PCR tests segment accounted for the largest global influenza diagnostics market share in 2022. RT-PCR boasts exceptional accuracy, detecting even trace amounts of viral RNA within hours. This rapid turnaround time (TAT) allows for prompt diagnosis and initiation of targeted treatment, improving patient outcomes and curbing transmission.
RT-PCR's ability to differentiate between influenza A and B subtypes is crucial for appropriate antiviral therapy and public health interventions. RT-PCR assays are readily scalable, adapting to high testing demands during outbreaks. Additionally, automation minimizes human error and increases throughput, crucial for managing large-scale testing needs.
Recognizing RT-PCR's advantages, governments worldwide are actively promoting its adoption. India's Integrated Disease Surveillance Programme (IDSP) emphasizes RT-PCR as the primary influenza diagnostic tool. This nationwide push fosters infrastructure development and trained personnel, further solidifying RT-PCR's dominance.
A 2020 study by the Centers for Disease Control and Prevention (CDC) found that RT-PCR had a 98.7% sensitivity and 99.5% specificity for influenza A and B detection, compared to 78.1% and 96.6% for rapid influenza diagnostic tests (RIDTs).
As per influenza diagnostics market analysis, North America dominated the global industry in 2022. This is ascribed to high disease burden, advanced healthcare infrastructure, stringent regulations, robust public health surveillance, and a supportive private and public sector landscape.
The U.S., with an average of 13.5 million annual influenza cases, bears the brunt of the disease in the region. Well-equipped hospitals and laboratories, coupled with widespread adoption of electronic health records, facilitate the use of sophisticated diagnostic tools such as molecular assays and rapid influenza diagnostic tests (RIDTs).
According to influenza diagnostics market forecast, the industry in Asia Pacific is projected to grow at a rapid pace during the forecast period. This is ascribed to rise in influenza burden, increase in healthcare awareness, expanding diagnostic options, aging population, and proactive government initiatives.
This growth trajectory holds immense potential for further development and innovation in influenza diagnostics within the region, ultimately contributing to improved public health outcomes.
Leading players in the global market have adopted strategies such as mergers & acquisitions, strategic collaborations, and new product launches to expand presence and gain market share.
3M, Abbott, F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, BIOMÉRIEUX, Bio-Rad Laboratories, Inc., Goldsite Diagnostics, Inc., Hologic, Inc., DiaSorin S.p.A., Meridian Bioscience, Inc., QuidelOrtho Corporation, SA Scientific Ltd., Sekisui Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific are the prominent players in the global influenza diagnostics market.
The influenza diagnostics market report profiles the top players based on various factors including company overview, financial summary, strategies, product portfolio, segments, and recent advancements.
| Attribute | Detail |
|---|---|
| Size in 2022 | US$ 1.3 Bn |
| Forecast (Value) in 2031 | More than US$ 2.0 Bn |
| Growth Rate (CAGR) | 5.6% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2022 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional-level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 1.3 Bn in 2022
It is projected to reach more than US$ 2.0 Bn by 2031
The CAGR is anticipated to be 5.6% from 2023 to 2031
The RT-PCR tests segment accounted for the largest share in 2022
North America is anticipated to account for the leading share during the forecast period.
3M, Abbott, F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, BIOMÉRIEUX, Bio-Rad Laboratories, Inc., Goldsite Diagnostics, Inc., Hologic, Inc., DiaSorin S.p.A., Meridian Bioscience, Inc., QuidelOrtho Corporation, SA Scientific Ltd., Sekisui Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Influenza Diagnostics Market
4. Market Overview
4.1. Introduction
4.1.1. Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Influenza Diagnostics Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Disease Epidemiology
5.2. Reimbursement Scenario
5.3. COVID-19 Pandemic Impact on Industry
6. Global Influenza Diagnostics Market Analysis and Forecast, by Flu Virus Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Flu Virus Type, 2017–2031
6.3.1. Type A
6.3.2. Type B
6.4. Market Attractiveness Analysis, by Flu Virus Type
7. Global Influenza Diagnostics Market Analysis and Forecast, by Test Type
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Test Type, 2017–2031
7.3.1. RT-PCR Tests
7.3.2. Rapid Influenza Diagnostic Tests (RIDTs)
7.3.3. Viral Culture
7.3.4. Immunofluorescence Assays
7.3.5. Serological Tests
7.3.6. Others (LAMP, SAMBA, etc.)
7.4. Market Attractiveness Analysis, by Test Type
8. Global Influenza Diagnostics Market Analysis and Forecast, by Product Type
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Product Type, 2017–2031
8.3.1. Kits & Reagents
8.3.2. Instrument
8.3.3. Others
8.4. Market Attractiveness Analysis, by Product Type
9. Global Influenza Diagnostics Market Analysis and Forecast, by End-user
9.1. Introduction & Definition
9.2. Key Findings / Developments
9.3. Market Value Forecast, by End-user, 2017–2031
9.3.1. Hospitals
9.3.2. Diagnostic Centers
9.3.3. Others (academics & research institutes, etc.)
9.4. Market Attractiveness Analysis, by End-user
10. Global Influenza Diagnostics Market Analysis and Forecast, by Region
10.1. Key Findings
10.2. Market Value Forecast, by Region
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Market Attractiveness Analysis, by Region
11. North America Influenza Diagnostics Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Flu Virus Type, 2017–2031
11.2.1. Type A
11.2.2. Type B
11.3. Market Value Forecast, by Test Type, 2017–2031
11.3.1. RT-PCR Tests
11.3.2. Rapid Influenza Diagnostic Tests (RIDTs)
11.3.3. Viral Culture
11.3.4. Immunofluorescence Assays
11.3.5. Serological Tests
11.3.6. Others (LAMP, SAMBA, etc.)
11.4. Market Value Forecast, by Product Type, 2017–2031
11.4.1. Kits & Reagents
11.4.2. Instrument
11.4.3. Others
11.5. Market Value Forecast, by End-user, 2017–2031
11.5.1. Hospitals
11.5.2. Diagnostic Centers
11.5.3. Others (academics & research institutes, etc.)
11.6. Market Value Forecast, by Country, 2017–2031
11.6.1. U.S.
11.6.2. Canada
11.7. Market Attractiveness Analysis
11.7.1. By Flu Virus Type
11.7.2. By Test Type
11.7.3. By Product Type
11.7.4. By End-user
11.7.5. By Country
12. Europe Influenza Diagnostics Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Flu Virus Type, 2017–2031
12.2.1. Type A
12.2.2. Type B
12.3. Market Value Forecast, by Test Type, 2017–2031
12.3.1. RT-PCR Tests
12.3.2. Rapid Influenza Diagnostic Tests (RIDTs)
12.3.3. Viral Culture
12.3.4. Immunofluorescence Assays
12.3.5. Serological Tests
12.3.6. Others (LAMP, SAMBA, etc.)
12.4. Market Value Forecast, by Product Type, 2017–2031
12.4.1. Kits & Reagents
12.4.2. Instrument
12.4.3. Others
12.5. Market Value Forecast, by End-user, 2017–2031
12.5.1. Hospitals
12.5.2. Diagnostic Centers
12.5.3. Others (academics & research institutes, etc.)
12.6. Market Value Forecast, by Country/Sub-region, 2017–2031
12.6.1. Germany
12.6.2. U.K.
12.6.3. France
12.6.4. Spain
12.6.5. Italy
12.6.6. Rest of Europe
12.7. Market Attractiveness Analysis
12.7.1. By Flu Virus Type
12.7.2. By Test Type
12.7.3. By Product Type
12.7.4. By End-user
12.7.5. By Country/Sub-region
13. Asia Pacific Influenza Diagnostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Flu Virus Type, 2017–2031
13.2.1. Type A
13.2.2. Type B
13.3. Market Value Forecast, by Test Type, 2017–2031
13.3.1. RT-PCR Tests
13.3.2. Rapid Influenza Diagnostic Tests (RIDTs)
13.3.3. Viral Culture
13.3.4. Immunofluorescence Assays
13.3.5. Serological Tests
13.3.6. Others (LAMP, SAMBA, etc.)
13.4. Market Value Forecast, by Product Type, 2017–2031
13.4.1. Kits & Reagents
13.4.2. Instrument
13.4.3. Others
13.5. Market Value Forecast, by End-user, 2017–2031
13.5.1. Hospitals
13.5.2. Diagnostic Centers
13.5.3. Others (academics & research institutes, etc.)
13.6. Market Value Forecast, by Country/Sub-region, 2017–2031
13.6.1. China
13.6.2. Japan
13.6.3. India
13.6.4. Australia & New Zealand
13.6.5. Rest of Asia Pacific
13.7. Market Attractiveness Analysis
13.7.1. By Flu Virus Type
13.7.2. By Test Type
13.7.3. By Product Type
13.7.4. By End-user
13.7.5. By Country/Sub-region
14. Latin America Influenza Diagnostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Flu Virus Type, 2017–2031
14.2.1. Type A
14.2.2. Type B
14.3. Market Value Forecast, by Test Type, 2017–2031
14.3.1. RT-PCR Tests
14.3.2. Rapid Influenza Diagnostic Tests (RIDTs)
14.3.3. Viral Culture
14.3.4. Immunofluorescence Assays
14.3.5. Serological Tests
14.3.6. Others (LAMP, SAMBA, etc.)
14.4. Market Value Forecast, by Product Type, 2017–2031
14.4.1. Kits & Reagents
14.4.2. Instrument
14.4.3. Others
14.5. Market Value Forecast, by End-user, 2017–2031
14.5.1. Hospitals
14.5.2. Diagnostic Centers
14.5.3. Others (academics & research institutes, etc.)
14.6. Market Value Forecast, by Country/Sub-region, 2017–2031
14.6.1. Brazil
14.6.2. Mexico
14.6.3. Rest of Latin America
14.7. Market Attractiveness Analysis
14.7.1. By Flu Virus Type
14.7.2. By Test Type
14.7.3. By Product Type
14.7.4. By End-user
14.7.5. By Country/Sub-region
15. Middle East & Africa Influenza Diagnostics Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Flu Virus Type, 2017–2031
15.2.1. Type A
15.2.2. Type B
15.3. Market Value Forecast, by Test Type, 2017–2031
15.3.1. RT-PCR Tests
15.3.2. Rapid Influenza Diagnostic Tests (RIDTs)
15.3.3. Viral Culture
15.3.4. Immunofluorescence Assays
15.3.5. Serological Tests
15.3.6. Others (LAMP, SAMBA, etc.)
15.4. Market Value Forecast, by Product Type, 2017–2031
15.4.1. Kits & Reagents
15.4.2. Instrument
15.4.3. Others
15.5. Market Value Forecast, by End-user, 2017–2031
15.5.1. Hospitals
15.5.2. Diagnostic Centers
15.5.3. Others (academics & research institutes, etc.)
15.6. Market Value Forecast, by Country/Sub-region, 2017–2031
15.6.1. GCC Countries
15.6.2. South Africa
15.6.3. Rest of Middle East & Africa
15.7. Market Attractiveness Analysis
15.7.1. By Flu Virus Type
15.7.2. By Test Type
15.7.3. By Product Type
15.7.4. By End-user
15.7.5. By Country/Sub-region
16. Competition Landscape
16.1. Market Player – Competition Matrix (by tier and size of companies)
16.2. Market Share Analysis, by Company (2022)
16.3. Company Profiles
16.3.1. 3M
16.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.1.2. Product Portfolio
16.3.1.3. Financial Overview
16.3.1.4. SWOT Analysis
16.3.1.5. Strategic Overview
16.3.2. Abbott
16.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.2.2. Product Portfolio
16.3.2.3. Financial Overview
16.3.2.4. SWOT Analysis
16.3.2.5. Strategic Overview
16.3.3. F. Hoffmann-La Roche Ltd.
16.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.3.2. Product Portfolio
16.3.3.3. Financial Overview
16.3.3.4. SWOT Analysis
16.3.3.5. Strategic Overview
16.3.4. Becton, Dickinson and Company
16.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.4.2. Product Portfolio
16.3.4.3. Financial Overview
16.3.4.4. SWOT Analysis
16.3.4.5. Strategic Overview
16.3.5. BIOMÉRIEUX
16.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.5.2. Product Portfolio
16.3.5.3. Financial Overview
16.3.5.4. SWOT Analysis
16.3.5.5. Strategic Overview
16.3.6. Bio-Rad Laboratories, Inc.
16.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.6.2. Product Portfolio
16.3.6.3. Financial Overview
16.3.6.4. SWOT Analysis
16.3.6.5. Strategic Overview
16.3.7. Goldsite Diagnostics Inc.
16.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.7.2. Product Portfolio
16.3.7.3. Financial Overview
16.3.7.4. SWOT Analysis
16.3.7.5. Strategic Overview
16.3.8. Hologic, Inc.
16.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.8.2. Product Portfolio
16.3.8.3. Financial Overview
16.3.8.4. SWOT Analysis
16.3.8.5. Strategic Overview
16.3.9. DiaSorin S.p.A.
16.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.9.2. Product Portfolio
16.3.9.3. Financial Overview
16.3.9.4. SWOT Analysis
16.3.9.5. Strategic Overview
16.3.10. Meridian Bioscience, Inc.
16.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.10.2. Product Portfolio
16.3.10.3. Financial Overview
16.3.10.4. SWOT Analysis
16.3.10.5. Strategic Overview
16.3.11. QuidelOrtho Corporation
16.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.11.2. Product Portfolio
16.3.11.3. Financial Overview
16.3.11.4. SWOT Analysis
16.3.11.5. Strategic Overview
16.3.12. SA Scientific Ltd.
16.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.12.2. Product Portfolio
16.3.12.3. Financial Overview
16.3.12.4. SWOT Analysis
16.3.12.5. Strategic Overview
16.3.13. Sekisui Diagnostics
16.3.13.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.13.2. Product Portfolio
16.3.13.3. Financial Overview
16.3.13.4. SWOT Analysis
16.3.13.5. Strategic Overview
16.3.14. Siemens Healthineers
16.3.14.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.14.2. Product Portfolio
16.3.14.3. Financial Overview
16.3.14.4. SWOT Analysis
16.3.14.5. Strategic Overview
16.3.15. Thermo Fisher Scientific
16.3.15.1. Company Overview (HQ, Business Segments, Employee Strength)
16.3.15.2. Product Portfolio
16.3.15.3. Financial Overview
16.3.15.4. SWOT Analysis
16.3.15.5. Strategic Overview
List of Tables
Table 01: Global Influenza Diagnostics Market Value (US$ Mn) Forecast, by Flu Virus Type, 2017-2031
Table 02: Global Influenza Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017-2031
Table 03: Global Influenza Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 04: Global Influenza Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 05: Global Influenza Diagnostics Market Value (US$ Mn) Forecast, by Region, 2017-2031
Table 06: North America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Country, 2017-2031
Table 07: North America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Flu Virus Type, 2017-2031
Table 08: North America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017-2031
Table 09: North America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 10: North America Influenza Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 11: Europe Influenza Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 12: Europe Influenza Diagnostics Market Value (US$ Mn) Forecast, by Flu Virus Type, 2017-2031
Table 13: Europe Influenza Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017-2031
Table 14: Europe Influenza Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 15: Europe Influenza Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 16: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 17: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Forecast, by Flu Virus Type, 2017-2031
Table 18: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017-2031
Table 19: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 20: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 21: Latin America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 22: Latin America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Flu Virus Type, 2017-2031
Table 23: Latin America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017-2031
Table 24: Latin America Influenza Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 25: Latin America Influenza Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 26: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 27: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Forecast, by Flu Virus Type, 2017-2031
Table 28: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017-2031
Table 29: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Forecast, by Product Type, 2017-2031
Table 30: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017-2031
List of Figures
Figure 01: Global Influenza Diagnostics Market Size, by Flu Virus Type, 2022
Figure 02: Global Influenza Diagnostics Market Share (%), by Flu Virus Type, 2022
Figure 03: Global Influenza Diagnostics Market Size, by Test Type, 2022
Figure 04: Global Influenza Diagnostics Market Share (%), by Test Type, 2022
Figure 05: Global Influenza Diagnostics Market Size, by Product Type, 2022
Figure 06: Global Influenza Diagnostics Market Share (%), by Product Type, 2022
Figure 07: Global Influenza Diagnostics Market Size, by End-user, 2022
Figure 08: Global Influenza Diagnostics Market Share (%), by End-user, 2022
Figure 09: Global Influenza Diagnostics Market, by Region (2022 and 2031)
Figure 10: Global Influenza Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 11: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022 and 2031
Figure 12: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022
Figure 13: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2031
Figure 14: Global Influenza Diagnostics Market Attractiveness Analysis, by Flu Virus Type, 2023–2031
Figure 15: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022 and 2031
Figure 16: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022
Figure 17: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2031
Figure 18: Global Influenza Diagnostics Market Attractiveness Analysis, by Test Type, 2023–2031
Figure 19: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022 and 2031
Figure 20: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022
Figure 21: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2031
Figure 22: Global Influenza Diagnostics Market Attractiveness Analysis, by Product Type, 2023–2031
Figure 23: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022 and 2031
Figure 24: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022
Figure 25: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2031
Figure 26: Global Influenza Diagnostics Market Attractiveness Analysis, by End-user, 2023–2031
Figure 27: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Region, 2022 and 2031
Figure 28: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Region, 2022
Figure 29: Global Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Region, 2031
Figure 30: Global Influenza Diagnostics Market Attractiveness Analysis, by Region, 2023–2031
Figure 31: North America Influenza Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017-2031
Figure 32: North America Influenza Diagnostics Market Value Share Analysis, by Country, 2022 and 2031
Figure 33: North America Influenza Diagnostics Market Attractiveness Analysis, by Country, 2023–2031
Figure 34: North America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022 and 2031
Figure 35: North America Influenza Diagnostics Market Attractiveness Analysis, by Flu Virus Type, 2023-2031
Figure 36: North America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022 and 2031
Figure 37: North America Influenza Diagnostics Market Attractiveness Analysis, by Test Type, 2023-31
Figure 38: North America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022 and 2031
Figure 39: North America Influenza Diagnostics Market Attractiveness Analysis, by Product Type, 2023-31
Figure 40: North America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022 and 2031
Figure 41: North America Influenza Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 42: Europe Influenza Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017-2031
Figure 43: Europe Influenza Diagnostics Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 44: Europe Influenza Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 45: Europe Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022 and 2031
Figure 46: Europe Influenza Diagnostics Market Attractiveness Analysis, by Flu Virus Type, 2023-2031
Figure 47: Europe Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022 and 2031
Figure 48: Europe Influenza Diagnostics Market Attractiveness Analysis, by Test Type, 2023-2031
Figure 49: Europe Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022 and 2031
Figure 50: Europe Influenza Diagnostics Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 51: Europe Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022 and 2031
Figure 52: Europe Influenza Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 53: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017-2031
Figure 54: Asia Pacific Influenza Diagnostics Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 55: Asia Pacific Influenza Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 56: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022 and 2031
Figure 57: Asia Pacific Influenza Diagnostics Market Attractiveness Analysis, by Flu Virus Type, 2023-2031
Figure 58: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022 and 2031
Figure 59: Asia Pacific Influenza Diagnostics Market Attractiveness Analysis, by Test Type, 2023-2031
Figure 60: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022 and 2031
Figure 61: Asia Pacific Influenza Diagnostics Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 62: Asia Pacific Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022 and 2031
Figure 63: Asia Pacific Influenza Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 64: Latin America Influenza Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017-2031
Figure 65: Latin America Influenza Diagnostics Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 66: Latin America Influenza Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 67: Latin America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022 and 2031
Figure 68: Latin America Influenza Diagnostics Market Attractiveness Analysis, by Flu Virus Type, 2023-2031
Figure 69: Latin America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022 and 2031
Figure 70: Latin America Influenza Diagnostics Market Attractiveness Analysis, by Test Type, 2023-2031
Figure 71: Latin America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022 and 2031
Figure 72: Latin America Influenza Diagnostics Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 73: Latin America Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022 and 2031
Figure 74: Latin America Influenza Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 75: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017-2031
Figure 76: Middle East & Africa Influenza Diagnostics Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 77: Middle East & Africa Influenza Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 78: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Flu Virus Type, 2022 and 2031
Figure 79: Middle East & Africa Influenza Diagnostics Market Attractiveness Analysis, by Flu Virus Type, 2023-2031
Figure 80: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Test Type, 2022 and 2031
Figure 81: Middle East & Africa Influenza Diagnostics Market Attractiveness Analysis, by Test Type, 2023-2031
Figure 82: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by Product Type, 2022 and 2031
Figure 83: Middle East & Africa Influenza Diagnostics Market Attractiveness Analysis, by Product Type, 2023-2031
Figure 84: Middle East & Africa Influenza Diagnostics Market Value (US$ Mn) Share Analysis, by End-user, 2022 and 2031
Figure 85: Middle East & Africa Influenza Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031