Reports
Reports
Vendors in the clinical trial data management software market have taken a step forward to join the fight against coronavirus (COVID-19). For instance, Castor EDC— a provider of CTMS tools on the centralized platform, announced to make its research data capture system available for free to meet the needs of users conducting COVID-19 research projects. Several companies in the clinical trial data management software market are continuing to deliver their services without any interruptions by adopting work from home and telecommunication policies.
The demand for clinical trial data management software is anticipated to surge, as research studies on COVID-19 are estimated to grow in number. Unprecedented demand for vaccines and coronavirus-fighting drugs is another key driver that is predicted to generate incremental opportunities for companies in the market landscape. Vendors are increasing the availability of eCRFs (Case Report Form) that help researchers start their study and registry in less than an hour.
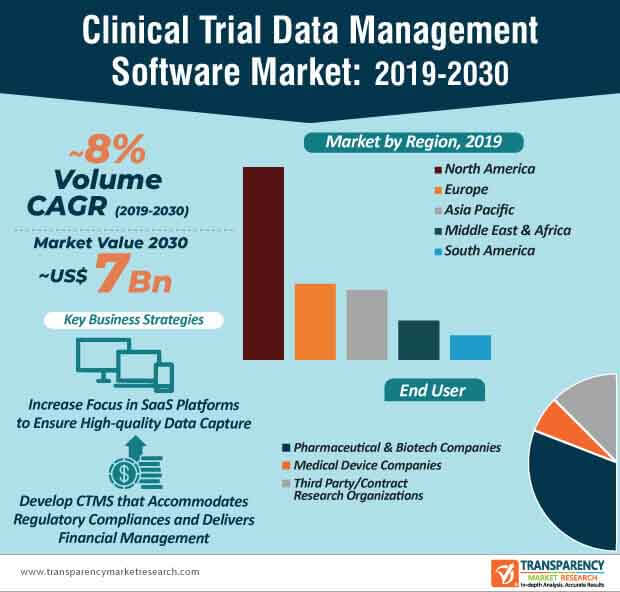
Software as a Service (SaaS) is emerging as a profitable investment option for pharmaceutical and biotech companies in the field of clinical trial data management software. For instance, leading information technology (IT) consulting TATA Consultancy Services is increasing awareness about its innovative SaaS platform, the TCS Connected Clinical TrialsTM that helps pharmaceutical companies in personalized patient engagement. The demand for high-quality data capture is rising in the clinical trial data management software market.
SaaS-inspired clinical trial data management software are becoming increasingly mainstream in pharmaceutical and biotech companies. This is evident since pharmaceutical and biotech companies are estimated to generate the highest revenue among all end users in the clinical trial data management software market, where the market is estimated to reach a value of ~US$ 7 Bn by the end of 2030. Clinical trial management software (CTMS) is being highly publicized for improving operational efficiency and compliance, while conducting clinical trials.
Companies in the clinical trial data management software market are increasing their focus to meet the needs of third party end users and contract research organizations (CROs). For instance, Veeva Systems— a leading U.S. cloud-computing company is serving the need of CROs by offering their Veeva Vault Clinical Data Management Suite (CDMS) that deploys greater visibility and control over data. As such, the revenue of third party/CRO end users is projected for exponential growth in the clinical trial data management software market, where the market is estimated to advance at a healthy CAGR of ~8% throughout the forecast period.
There is a growing demand for clinical trial data management software that eliminates manual processes and accelerates data processing capabilities. Next-gen interfaces in CDMS solutions are helping clinical research associates (CRA) to accurately review data and eliminates the need to page through forms. Vendors are increasing efforts to deliver better electronic data capture (EDC) with the help of clinical trial data management software in complex and multi-arm adaptive trials.
Most researchers are familiar with Excel, Google Forms, SPSS (Statistical Package for the Social Sciences), and the likes for data capture in the clinical trial data management software market. However, these tools are cumbersome and cannot be efficiently deployed for medical data capture. Hence, vendors are increasing awareness about clinical trial data management software to meet the needs of medical device companies. However, loss of productivity levels due to the fresh adoption of software is a shortcoming that vendors need to tackle. Hence, companies are innovating in EDC platforms that are specially meant for medical device and other healthcare companies.
Companies in the market for clinical trial data management software are gaining efficacy in developing multi-purpose tools for EDC. EDC tools are gaining increased popularity in the clinical trial data management software market, since they eliminate the need to have programming knowledge, thus boosting its adoption among small-scale medical research companies.

Analysts’ Viewpoint
Data is increasingly becoming a valuable asset amidst the coronavirus outbreak, as data capture systems have decades of stored information related to COVID-19 strains. SaaS platforms are being increasingly used to improve the efficiency and accountability of clinical trial processes.
CTMS solutions are gaining prominence in financial management and in the management of multiple studies. However, several EDC systems demand the need for programming knowledge for its adoption in medical research settings. Hence, companies in the clinical trial data management software market should increase the availability of EDC tools that are specially meant for medical research and are capable of performing advanced calculations without any prior experience.
1. Preface
1.1. Market Introduction
1.2. Market Segmentation
1.3. Key Research Objectives
2. Assumptions and Research Methodology
2.1. Research Methodology
2.1.1. List of Primary and Secondary Sources
2.2. Key Assumptions for Data Modelling
3. Executive Summary - Global Clinical Trial Data Management Software Market
4. Market Overview
4.1. Market Definition
4.2. Macroeconomic Factors
4.2.1. World GDP Indicator – For Top Economies
4.2.2. Global ICT Spending (US$ Mn)
4.3. Technology/ Product Roadmap
4.4. Market Factor Analysis
4.4.1. Porter’s Five Forces Analysis
4.4.2. Ecosystem/ Value Chain Analysis
4.4.3. Market Dynamics (Growth Influencers)
4.4.3.1. Drivers
4.4.3.2. Restraints
4.4.3.3. Opportunities
4.4.3.4. Impact Analysis of Drivers and Restraints
4.5. Market Opportunity Assessment – by Region (North America/ Europe/ Asia Pacific/ Middle East & Africa/ South America)
4.5.1. By Component
4.5.2. By End-user
4.6. Competitive Scenario and Trends
4.6.1. Clinical Trial Data Management Software Market Concentration Rate
4.6.1.1. List of Emerging, Prominent and Leading Players
4.6.2. Mergers & Acquisitions, Expansions
4.7. Market Outlook
4.8. Impact Analysis of COVID-19 on Clinical Trial Data Management Software Market
5. Global Clinical Trial Data Management Software Market Analysis and Forecast
5.1.1. Market Revenue Analysis (US$ Mn), 2015-2030
5.1.1.1. Historic Growth Trends, 2015-2019
5.1.1.2. Forecast Trends, 2020-2030
6. Global Clinical Trial Data Management Software Market Analysis, by Component
6.1. Overview and Definitions
6.2. Key Segment Analysis
6.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Component, 2018 - 2030
6.3.1. Software
6.3.1.1. On-Premise/ Enterprise
6.3.1.2. On Demand Software as a Service (SaaS)
6.3.2. Services
6.3.2.1. Professional
6.3.2.2. Managed
7. Global Clinical Trial Data Management Software Market Analysis, by End-user
7.1. Overview
7.2. Key Segment Analysis
7.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by End-user, 2018 - 2030
7.3.1. Pharmaceutical & Biotech Companies
7.3.2. Medical Device Companies
7.3.3. Third Party/ Contract Research Organizations
8. Global Clinical Trial Data Management Software Market Analysis and Forecast, By Region
8.1. Key Findings
8.2. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Region, 2018 - 2030
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Middle East & Africa
8.2.5. South America
9. North America Clinical Trial Data Management Software Market Analysis
9.1. Regional Outlook
9.2. Clinical Trial Data Management Software Market Size (US$ Mn) Analysis and Forecast (2018 - 2030)
9.2.1. By Component
9.2.2. By End-user
9.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Country, 2018 - 2030
9.3.1. U.S.
9.3.2. Canada
9.3.3. Mexico
10. Europe Clinical Trial Data Management Software Market Analysis and Forecast
10.1. Regional Outlook
10.2. Clinical Trial Data Management Software Market Size (US$ Mn) Analysis and Forecast (2018 - 2030)
10.2.1. By Component
10.2.2. By End-user
10.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Country & Sub-region, 2018 - 2030
10.3.1. Germany
10.3.2. U.K.
10.3.3. France
10.3.4. Spain
10.3.5. Italy
10.3.6. Rest of Europe
11. APAC Clinical Trial Data Management Software Market Analysis and Forecast
11.1. Regional Outlook
11.2. Clinical Trial Data Management Software Market Size (US$ Mn) Analysis and Forecast (2018 - 2030)
11.2.1. By Component
11.2.2. By End-user
11.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Country & Sub-region, 2018 - 2030
11.3.1. China
11.3.2. India
11.3.3. Japan
11.3.4. ASEAN
11.3.5. Rest of Asia Pacific
12. Middle East & Africa (MEA) Clinical Trial Data Management Software Market Analysis and Forecast
12.1. Regional Outlook
12.2. Clinical Trial Data Management Software Market Size (US$ Mn) Analysis and Forecast (2018 - 2030)
12.2.1. By Component
12.2.2. By End-user
12.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Country & Sub-region, 2018 - 2030
12.3.1. Saudi Arabia
12.3.2. Kuwait
12.3.3. The United Arab Emirates
12.3.4. Qatar
12.3.5. Bahrain
12.3.6. Oman
12.3.7. South Africa
12.3.8. Rest of Middle East & Africa
13. South America Clinical Trial Data Management Software Market Analysis and Forecast
13.1. Regional Outlook
13.2. Clinical Trial Data Management Software Market Size (US$ Mn) Analysis and Forecast (2018 - 2030)
13.2.1. By Component
13.2.2. By End-user
13.3. Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, by Country & Sub-region, 2018 - 2030
13.3.1. Brazil
13.3.2. Argentina
13.3.3. Rest of South America
14. Competition Landscape
14.1. Market Competition Matrix, by Leading Players
14.2. Market Revenue Share Analysis (%), by Leading Players (2019)
15. Company Profiles
15.1. Bioclinica
15.1.1. Business Overview
15.1.2. Product Portfolio
15.2. Bio-Optronics
15.2.1. Business Overview
15.2.2. Product Portfolio
15.3. Forte Research Systems
15.3.1. Business Overview
15.3.2. Product Portfolio
15.4. IBM Corporation
15.4.1. Business Overview
15.4.2. Product Portfolio
15.4.3. Geographical Footprint
15.4.4. Revenue and Strategy
15.5. Medidata Solutions
15.5.1. Business Overview
15.5.2. Product Portfolio
15.5.3. Geographical Footprint
15.5.4. Revenue and Strategy
15.6. Oracle Corporation
15.6.1. Business Overview
15.6.2. Product Portfolio
15.6.3. Geographical Footprint
15.6.4. Revenue and Strategy
15.7. Parexel
15.7.1. Business Overview
15.7.2. Product Portfolio
15.8. Quad One Technologies Pvt. Ltd.
15.8.1. Business Overview
15.8.2. Product Portfolio
15.9. Trial By Fire Solutions
15.9.1. Business Overview
15.9.2. Product Portfolio
15.10. Veeva Systems Inc.
15.10.1. Business Overview
15.10.2. Product Portfolio
15.10.3. Geographical Footprint
15.10.4. Revenue and Strategy
16. Key Takeaways
List of Tables
Table 1: Global Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, 2018 - 2030
Table 2: Global Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Software, 2018 - 2030
Table 3: Global Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Services, 2018 - 2030
Table 4: Global Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by End-user, 2018 - 2030
Table 5: Global Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Region, 2018 - 2030
Table 6: North America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, 2018 - 2030
Table 7: North America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Software, 2018 - 2030
Table 8: North America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Services, 2018 - 2030
Table 9: North America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by End-user, 2018 - 2030
Table 10: North America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Country, 2018 - 2030
Table 11: Europe Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, 2018 - 2030
Table 12: Europe Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Software, 2018 - 2030
Table 13: Europe Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Services, 2018 - 2030
Table 14: Europe Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by End-user, 2018 - 2030
Table 15: Europe Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Country, 2018 - 2030
Table 16: Asia Pacific Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, 2018 - 2030
Table 17: Asia Pacific Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Software, 2018 - 2030
Table 18: Asia Pacific Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Services, 2018 - 2030
Table 19: Asia Pacific Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by End-user, 2018 - 2030
Table 20: Asia Pacific Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Country, 2018 - 2030
Table 21: Middle East & Africa Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, 2018 - 2030
Table 22: Middle East & Africa Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Software, 2018 - 2030
Table 23: Middle East & Africa Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Services, 2018 - 2030
Table 24: Middle East & Africa Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by End-user, 2018 - 2030
Table 25: Middle East & Africa Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Country, 2018 - 2030
Table 26: South America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, 2018 - 2030
Table 27: South America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Software, 2018 - 2030
Table 28: South America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Component, by Services, 2018 - 2030
Table 29: South America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by End-user, 2018 - 2030
Table 30: South America Clinical Trial Data Management Software Market Value (US$ Mn) Forecast, by Country, 2018 - 2030
List of Figures
Figure 1: Global Clinical Trial Data Management Software Market Size (US$ Mn) Forecast, 2018 – 2030
Figure 2: Global Clinical Trial Data Management Software Market Revenue (US$ Mn) Opportunity Assessment, by Region, 2020E
Figure 3: Top Segment Analysis
Figure 4: Global Clinical Trial Data Management Software Market Revenue (US$ Mn) Opportunity Assessment, by Region, 2030F
Figure 5: GDP (US$ Bn), Top Countries (2014 – 2019)
Figure 6: Top Economies GDP Landscape, 2019
Figure 7: Global ICT Spending (%), by Region, 2019
Figure 8: Global ICT Spending (US$ Bn), Regional Contribution, 2019
Figure 9: Global ICT Spending (US$ Bn), Spending Type Contribution, 2019
Figure 10: Global ICT Spending (%), by Type, 2019
Figure 11: Global Clinical Trial Data Management Software Market Attractiveness Assessment, by Component
Figure 12: Global Clinical Trial Data Management Software Market Relative Attractiveness Assessment, by Component
Figure 13: Global Clinical Trial Data Management Software Market Attractiveness Assessment, by End-user
Figure 14: Global Clinical Trial Data Management Software Market Relative Attractiveness Assessment, by End-user
Figure 15: Global Clinical Trial Data Management Software Market Attractiveness Assessment, by Region
Figure 16: Global Clinical Trial Data Management Software Market Relative Attractiveness Assessment, by Region
Figure 17: Global Clinical Trial Data Management Software Market, by Component, CAGR (%) (2020 – 2030)
Figure 18: Global Clinical Trial Data Management Software Market, by End-user, CAGR (%) (2020 – 2030)
Figure 19: Global Clinical Trial Data Management Software Market, by Region, CAGR (%) (2020 – 2030)
Figure 20: Global Clinical Trial Data Management Software Market Revenue (US$ Mn) Historic Trends, 2015 - 2019
Figure 21: Global Clinical Trial Data Management Software Market Revenue Opportunity (US$ Mn) Historic Trends, 2015 - 2019
Figure 22: Global Clinical Trial Data Management Software Market Revenue (US$ Mn) and Y-o-Y Growth (Revenue %) Forecast, 2020 - 2030
Figure 23: Global Clinical Trial Data Management Software Market Revenue Opportunity (US$ Mn) Forecast, 2020 - 2030
Figure 24: Global Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2020
Figure 25: Global Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2030
Figure 26: Global Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2020
Figure 27: Global Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2030
Figure 28: Global Clinical Trial Data Management Software Market Value Share Analysis, by Region, 2020
Figure 29: Global Clinical Trial Data Management Software Market Value Share Analysis, by Region, 2030
Figure 30: North America Clinical Trial Data Management Software Market Opportunity Growth Analysis (US$ Mn) Forecast, 2019 – 2030
Figure 31: North America Clinical Trial Data Management Software Market Y-o-Y Growth (Value %), 2019 - 2030
Figure 32: North America Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2020
Figure 33: North America Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2030
Figure 34: North America Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2020
Figure 35: North America Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2030
Figure 36: North America Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2020
Figure 37: North America Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2030
Figure 38: Europe Clinical Trial Data Management Software Market Opportunity Growth Analysis (US$ Mn) Forecast, 2019 – 2030
Figure 39: Europe Clinical Trial Data Management Software Market Y-o-Y Growth (Value %), 2019 - 2030
Figure 40: Europe Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2020
Figure 41: Europe Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2030
Figure 42: Europe Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2020
Figure 43: Europe Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2030
Figure 44: Europe Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2020
Figure 45: Europe Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2030
Figure 46: Asia Pacific Clinical Trial Data Management Software Market Opportunity Growth Analysis (US$ Mn) Forecast, 2019 – 2030
Figure 47: Asia Pacific Clinical Trial Data Management Software Market Y-o-Y Growth (Value %), 2019 - 2030
Figure 48: Asia Pacific Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2020
Figure 49: Asia Pacific Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2030
Figure 50: Asia Pacific Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2020
Figure 51: Asia Pacific Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2030
Figure 52: Asia Pacific Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2020
Figure 53: Asia Pacific Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2030
Figure 54: Middle East & Africa Clinical Trial Data Management Software Market Opportunity Growth Analysis (US$ Mn) Forecast, 2019 – 2030
Figure 55: Middle East & Africa Clinical Trial Data Management Software Market Y-o-Y Growth (Value %), 2019 - 2030
Figure 56: Middle East & Africa Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2020
Figure 57: Middle East & Africa Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2030
Figure 58: Middle East & Africa Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2020
Figure 59: Middle East & Africa Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2030
Figure 60: Middle East & Africa Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2020
Figure 61: Middle East & Africa Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2030
Figure 62: South America Clinical Trial Data Management Software Market Opportunity Growth Analysis (US$ Mn) Forecast, 2019 – 2030
Figure 63: South America Clinical Trial Data Management Software Market Y-o-Y Growth (Value %), 2019 - 2030
Figure 64: South America Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2020
Figure 65: South America Clinical Trial Data Management Software Market Value Share Analysis, by Component, 2030
Figure 66: South America Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2020
Figure 67: South America Clinical Trial Data Management Software Market Value Share Analysis, by End-user, 2030
Figure 68: South America Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2020
Figure 69: South America Clinical Trial Data Management Software Market Value Share Analysis, by Country, 2030
Figure 70: Market Revenue Share Analysis (%), by Leading Players (2019)