Analysts’ Viewpoint
A medical device undergoes testing, documentation, and quality control before it reaches the end-user. Medical device labeling is employed at every stage of the entire production and distribution process in order to properly manage the high volume of manufactured devices or drugs. The ease of handling devices and drugs offered by labeling units is expected to boost the medical device labeling industry growth during the forecast period.
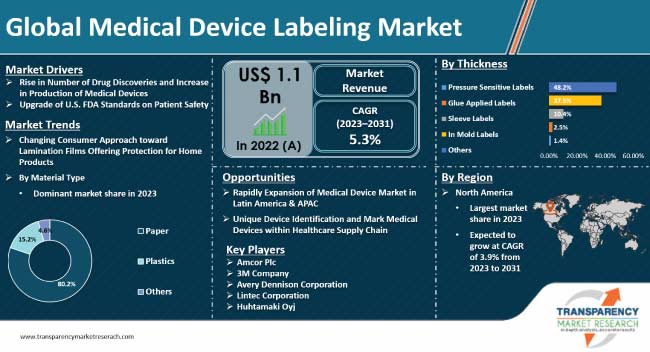
Stringent regulations enacted by the U.S. FDA on medical device manufacturers in the healthcare sector is driving the need for medical device labeling to segregate approved, non-approved, and recalled devices. This, in turn, is projected to create lucrative medical device labeling market opportunities for manufacturers during the forecast period.
Medical device manufacturers use medical device labeling to provide a general description of the medical device, apart from additional information such as the certifications by the U.S. FDA. Currently, manufacturers are also utilizing floating medical device labels with multi-lingual instructions in order to help end-users in various regions worldwide.
One of the notable medical device labeling market trends is the demand for medical device labels to include unique device identification (UDI) ‒ a unique alphanumeric or numeric code comprising two parts: production identifier (PI) and a device identifier (DI). However, stringent regulations on medical device labeling about compliance with the U.S. FDA could lead to rework and loss of production, which is likely to restrain the medical device labeling market development during the forecast period.
Demand for personal protective equipment and medical devices has been rising for the last few years due to increase in healthcare expenditure in developing world and rise in demand from aging populations in both developed and developing countries. Moreover, rise in the number of inpatient admissions and the increasing number of surgical and diagnostic procedures is fueling the demand for medical devices.
Efficient medical devices improve patient outcomes and lower overall cost on the part of healthcare. Medical device production needs to comply with stringent regulations that ensure products are effective and safe for patient use. Therefore, rise in number of drug discoveries is likely to fuel the demand for compliance with regulatory requirements, which include quality control, documentation, and production testing. All these steps need labeling units for smooth execution. These factors are projected to boost growth of the medical device labeling industry during the forecast period.
The U.S. FDA consistently upgrades its Code of Regulations that govern the matter written or printed labels of medical products. Medical labeling provides patients, industry, healthcare providers, and the public guidelines about medical devices and their usage. For instance, On November 30, 2023, the U.S. FDA informed healthcare providers, consumers, and healthcare facilities that it is evaluating potential for failures, such as breakage, leaks, and the other problems, of plastic syringes produced in China.
Manufacturers of devices and diagnostics across the globe are developing new products to improve quality of life of patients. Medical equipment labels and instructions-for-use (IFUs) play a critical role in boosting consumer confidence and developing trust between healthcare practitioners and patients. Therefore, upgrade of guidelines and regulations owing to development of various medical devices in order to improve patient safety across the globe is likely to propel the medical device labeling market dynamics in the next few years.
North America held a prominent medical device labeling market share in 2022 due to significant expansion of the healthcare industry and rise in investment in research & development in drug development in the region. Moreover, rise in adoption of home healthcare medical devices and adoption of UDI in medical device labeling are key factors that are likely to positively influence the medical device labeling market forecast in North America in the next few years.
The medical device labeling industry in Asia Pacific is expected to grow at a considerable pace during the forecast period due to significant expansion of the pharmaceutical manufacturing hubs in China and India. Furthermore, rise in spending on expansion of healthcare services in developing countries in Asia Pacific is estimated to boost the medical device labeling market revenue in the near future.
The global medical labeling industry is highly consolidated owing to the presence of numerous players with worldwide presence. Leading medical device labeling manufacturers are investing in rebranding, centralizing their enterprise systems, and standardizing their labeling lifecycle. Moreover, medical device manufacturers are exploring sustainable packaging solutions to tackle biomedical waste rampant across the industry.
3M Company, Amcor Ltd., Mondi Group plc, Avery Dennison Corporation, Huhtamaki Group, CCL Industries, Inc., LINTEC Corporation, UPM Raflatac, WS Packaging Group, Inc., Schreiner Group GmbH & co. KG, Resource label Group LLC, Faubel & Co. Nachf. GmbH, JH Bertrand Inc., Denny Bros Ltd., Tapecon Inc., Weber Packaging Solutions, Coast Label Company, and Label Source are a few prominent entities operating in the global market.
Key players in the medical device labeling market report have been profiled based on various parameters such as company overview, business strategies, financial overview, product portfolio, and business segments.
| Attribute | Detail |
|---|---|
| Size in 2022 | US$ 1.1 Bn |
| Forecast (Value) in 2031 | US$ 1.9 Bn |
| Growth Rate (CAGR) | 5.3% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2021 |
| Quantitative Units | US$ Bn for Value and Square Meters for Volume |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Market Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available Upon Request |
| Pricing | Available Upon Request |
The global market was valued at US$ 1.1 Bn in 2023
It is projected to grow at a CAGR of 5.3% from 2023 to 2031
Rise in number of drug discoveries and increase in production of medical devices and upgrade of FDA standards on patient safety
In terms of material type, the paper segment held largest share in 2022
North America is estimated to dominate in the next few years
3M Company, Amcor Ltd., Mondi Group plc, Avery Dennison Corporation, Huhtamaki Group, CCL Industries, Inc., Lintec Corporation, UPM Raflatac, WS Packaging Group, Inc., Schreiner Group GmbH & co. KG, Resource label Group LLC, Faubel & Co. Nachf. GmbH, JH Bertrand Inc., Denny Bros Ltd., Tapecon Inc., Weber Packaging Solutions, Coast Label Company, and Label Source
1. Executive Summary
1.1. Market Overview
1.2. Market Analysis
1.3. TMR Analysis and Recommendations
2. Market Viewpoint
2.1. Market Definition
2.2. Market Taxonomy
3. Medical Device Labeling Market Overview
3.1. Global Packaging Market Overview
3.2. Global Label Market Outlook
3.3. Macro-economic Factors – Correlation Analysis
3.4. Forecast Factors – Relevance & Impact
3.5. Medical Device Labeling Market Value Chain Analysis
3.5.1. Exhaustive List of Active Participants
3.5.1.1. Raw Label Suppliers
3.5.1.2. Manufactures
3.5.1.3. Distributors/ Retailers
3.5.2. Profitability Margins
3.6. Cased Based Scenario Impact Analysis
3.7. Market Dynamics
3.7.1. Drivers
3.7.2. Restraints
3.7.3. Opportunity Analysis
3.7.4. Trends
4. Medical Device Labeling Market Analysis
4.1. Pricing Analysis
4.1.1. Pricing Assumption
4.1.2. Price Projections By Region
4.2. Market Size (US$ Mn) and Forecast
4.2.1. Market Size and Y-o-Y Growth
4.2.2. Absolute $ Opportunity
5. Global Medical Device Labeling Market Analysis and Forecast, By Label Type
5.1. Introduction
5.1.1. Market share and Basis Points (BPS) Analysis, By Label Type
5.1.2. Y-o-Y Growth Projections, By Label Type
5.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Label Type
5.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Label Type
5.3.1. Pressure Sensitive Label
5.3.2. Glue Applied Labels
5.3.3. Sleeve Labels
5.3.4. In Mold Labels
5.3.5. Others
5.4. Market Attractiveness Analysis, By Label Type
6. Global Medical Device Labeling Market Analysis and Forecast, By Material Type
6.1. Introduction
6.1.1. Market share and Basis Points (BPS) Analysis, By Material Type
6.1.2. Y-o-Y Growth Projections, By Material Type
6.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Material Type
6.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Material Type
6.3.1. Paper
6.3.2. Plastic
6.3.3. Others
6.4. Market Attractiveness Analysis, By Material Type
7. Global Medical Device Labeling Market Analysis and Forecast, By Application
7.1. Introduction
7.1.1. Market share and Basis Points (BPS) Analysis, By Application
7.1.2. Y-o-Y Growth Projections, By Application
7.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Application
7.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Application
7.3.1. Disposable Consumables
7.3.2. Monitoring & Diagnostic Equipment
7.3.3. Therapeutic Equipment
7.4. Market Attractiveness Analysis, By Application
7.5. Prominent Trends
8. Global Medical Device Labeling Market Analysis and Forecast, By Region
8.1. Introduction
8.1.1. Market share and Basis Points (BPS) Analysis By Region
8.1.2. Y-o-Y Growth Projections By Region
8.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Region
8.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031 By Region
8.3.1. North America
8.3.2. Latin America
8.3.3. Europe
8.3.4. Asia Pacific
8.3.5. Middle East & Africa (MEA)
8.4. Market Attractiveness Analysis By Region
8.5. Prominent Trends
9. North America Medical Device Labeling Market Analysis and Forecast
9.1. Introduction
9.1.1. Market share and Basis Points (BPS) Analysis, By Country
9.1.2. Y-o-Y Growth Projections, By Country
9.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Country
9.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Country
9.3.1. U.S.
9.3.2. Canada
9.4. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Label Type
9.5. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Label Type
9.5.1. Pressure Sensitive Label
9.5.2. Glue Applied Labels
9.5.3. Sleeve Labels
9.5.4. In Mold Labels
9.5.5. Others
9.6. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Material Type
9.7. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Material Type
9.7.1. Paper
9.7.2. Plastic
9.7.3. Others
9.8. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Application
9.9. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Application
9.9.1. Disposable Consumables
9.9.2. Monitoring & Diagnostic Equipment
9.9.3. Therapeutic Equipment
9.10. Prominent Trends
9.11. Drivers and Restraints: Impact Analysis
10. Latin America Medical Device Labeling Market Analysis and Forecast
10.1. Introduction
10.1.1. Market share and Basis Points (BPS) Analysis, By Country
10.1.2. Y-o-Y Growth Projections, By Country
10.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Country
10.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031 By Country
10.3.1. Brazil
10.3.2. Mexico
10.3.3. Argentina
10.3.4. Rest of Latin America
10.4. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Label Type
10.5. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Label Type
10.5.1. Pressure Sensitive Label
10.5.2. Glue Applied Labels
10.5.3. Sleeve Labels
10.5.4. In Mold Labels
10.5.5. Others
10.6. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Material Type
10.7. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Material Type
10.7.1. Paper
10.7.2. Plastic
10.7.3. Others
10.8. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Application
10.9. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Application
10.9.1. Disposable Consumables
10.9.2. Monitoring & Diagnostic Equipment
10.9.3. Therapeutic Equipment
10.10. Prominent Trends
10.11. Drivers and Restraints: Impact Analysis
11. Europe Medical Device Labeling Market Analysis and Forecast
11.1. Introduction
11.1.1. Market share and Basis Points (BPS) Analysis, By Country
11.1.2. Y-o-Y Growth Projections, By Country
11.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Country
11.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031 By Country
11.3.1. Germany
11.3.2. Spain
11.3.3. Italy
11.3.4. France
11.3.5. U.K.
11.3.6. BENELUX
11.3.7. Nordic
11.3.8. Russia
11.3.9. Poland
11.3.10. Rest of Europe
11.4. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Label Type
11.5. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Label Type
11.5.1. Pressure Sensitive Label
11.5.2. Glue Applied Labels
11.5.3. Sleeve Labels
11.5.4. In Mold Labels
11.5.5. Others
11.6. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Material Type
11.7. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Material Type
11.7.1. Paper
11.7.2. Plastic
11.7.3. Others
11.8. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Application
11.9. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Application
11.9.1. Disposable Consumables
11.9.2. Monitoring & Diagnostic Equipment
11.9.3. Therapeutic Equipment
11.10. Prominent Trends
11.11. Drivers and Restraints: Impact Analysis
12. Asia Pacific Medical Device Labeling Market Analysis and Forecast
12.1. Introduction
12.1.1. Market share and Basis Points (BPS) Analysis, By Country
12.1.2. Y-o-Y Growth Projections, By Country
12.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Country
12.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031 By Country
12.3.1. China
12.3.2. India
12.3.3. Japan
12.3.4. ASEAN
12.3.5. Australia and New Zealand
12.3.6. South Korea
12.3.7. Rest of APAC
12.4. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Label Type
12.5. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Label Type
12.5.1. Pressure Sensitive Label
12.5.2. Glue Applied Labels
12.5.3. Sleeve Labels
12.5.4. In Mold Labels
12.5.5. Others
12.6. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Material Type
12.7. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Material Type
12.7.1. Paper
12.7.2. Plastic
12.7.3. Others
12.8. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Application
12.9. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Application
12.9.1. Disposable Consumables
12.9.2. Monitoring & Diagnostic Equipment
12.9.3. Therapeutic Equipment
12.10. Prominent Trends
12.11. Drivers and Restraints: Impact Analysis
13. Middle East and Africa Medical Device Labeling Market Analysis and Forecast
13.1. Introduction
13.1.1. Market share and Basis Points (BPS) Analysis, By Country
13.1.2. Y-o-Y Growth Projections, By Country
13.2. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Country
13.3. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Country
13.3.1. North Africa
13.3.2. GCC countries
13.3.3. South Africa
13.3.4. Turkey
13.3.5. Rest of MEA
13.4. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Label Type
13.5. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Label Type
13.5.1. Pressure Sensitive Label
13.5.2. Glue Applied Labels
13.5.3. Sleeve Labels
13.5.4. In Mold Labels
13.5.5. Others
13.6. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Material Type
13.7. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Material Type
13.7.1. Paper
13.7.2. Plastic
13.7.3. Others
13.8. Historical Market Value(US$ Mn) and Volume (Square Meters), 2018-2022, By Application
13.9. Market Size (US$ Mn) and Volume (Square Meters) Forecast Analysis 2023-2031, By Application
13.9.1. Disposable Consumables
13.9.2. Monitoring & Diagnostic Equipment
13.9.3. Therapeutic Equipment
13.10. Prominent Trends
13.11. Drivers and Restraints: Impact Analysis
14. Competitive Landscape
14.1. Market Structure
14.2. Competition Dashboard
14.3. Company Market Share Analysis
14.4. Company Profiles (Details – Overview, Financials, Strategy, Recent Developments, SWOT analysis)
14.5. Competition Deep Dive
14.5.1. Amcor Limited
14.5.1.1. Overview
14.5.1.2. Financials
14.5.1.3. Strategy
14.5.1.4. Recent Developments
14.5.1.5. SWOT Analysis
14.5.2. 3M Company
14.5.2.1. Overview
14.5.2.2. Financials
14.5.2.3. Strategy
14.5.2.4. Recent Developments
14.5.2.5. SWOT Analysis
14.5.3. Avery Dennison Corporation
14.5.3.1. Overview
14.5.3.2. Financials
14.5.3.3. Strategy
14.5.3.4. Recent Developments
14.5.3.5. SWOT Analysis
14.5.4. Lintec Corporation
14.5.4.1. Overview
14.5.4.2. Financials
14.5.4.3. Strategy
14.5.4.4. Recent Developments
14.5.4.5. SWOT Analysis
14.5.5. Constantia Flexibles Group GmbH
14.5.5.1. Overview
14.5.5.2. Financials
14.5.5.3. Strategy
14.5.5.4. Recent Developments
14.5.5.5. SWOT Analysis
14.5.6. Huhtamaki Oyj
14.5.6.1. Overview
14.5.6.2. Financials
14.5.6.3. Strategy
14.5.6.4. Recent Developments
14.5.6.5. SWOT Analysis
14.5.7. UPM Raflatac
14.5.7.1. Overview
14.5.7.2. Financials
14.5.7.3. Strategy
14.5.7.4. Recent Developments
14.5.7.5. SWOT Analysis
14.5.8. Mondi Group plc.
14.5.8.1. Overview
14.5.8.2. Financials
14.5.8.3. Strategy
14.5.8.4. Recent Developments
14.5.8.5. SWOT Analysis
14.5.9. CCL Industries Inc.
14.5.9.1. Overview
14.5.9.2. Financials
14.5.9.3. Strategy
14.5.9.4. Recent Developments
14.5.9.5. SWOT Analysis
14.5.10. Schreiner Group GmbH & Co. KG
14.5.10.1. Overview
14.5.10.2. Financials
14.5.10.3. Strategy
14.5.10.4. Recent Developments
14.5.10.5. SWOT Analysis
14.5.11. Denny Bros Ltd.
14.5.11.1. Overview
14.5.11.2. Financials
14.5.11.3. Strategy
14.5.11.4. Recent Developments
14.5.11.5. SWOT Analysis
14.5.12. WS Packaging Group, Inc.
14.5.12.1. Overview
14.5.12.2. Financials
14.5.12.3. Strategy
14.5.12.4. Recent Developments
14.5.12.5. SWOT Analysis
14.5.13. Resource Label Group LLC
14.5.13.1. Overview
14.5.13.2. Financials
14.5.13.3. Strategy
14.5.13.4. Recent Developments
14.5.13.5. SWOT Analysis
14.5.14. Faubel & Co. Nachf. GmbH
14.5.14.1. Overview
14.5.14.2. Financials
14.5.14.3. Strategy
14.5.14.4. Recent Developments
14.5.14.5. SWOT Analysis
14.5.15. Tapecon Inc.
14.5.15.1. Overview
14.5.15.2. Financials
14.5.15.3. Strategy
14.5.15.4. Recent Developments
14.5.15.5. SWOT Analysis
14.5.16. Weber Packaging Solutions, Inc.
14.5.16.1. Overview
14.5.16.2. Financials
14.5.16.3. Strategy
14.5.16.4. Recent Developments
14.5.16.5. SWOT Analysis
14.5.17. JH Bertrand Inc.
14.5.17.1. Overview
14.5.17.2. Financials
14.5.17.3. Strategy
14.5.17.4. Recent Developments
14.5.17.5. SWOT Analysis
14.5.18. Label Source, Inc.
14.5.18.1. Overview
14.5.18.2. Financials
14.5.18.3. Strategy
14.5.18.4. Recent Developments
14.5.18.5. SWOT Analysis
14.5.19. Coast Label Company
14.5.19.1. Overview
14.5.19.2. Financials
14.5.19.3. Strategy
14.5.19.4. Recent Developments
14.5.19.5. SWOT Analysis
15. Assumptions and Acronyms Used
16. Research Methodology
List of Tables
Table 01: Global Medical Device Labeling Market Historic Value (US$ Mn), by Label Type 2018(H)-2022(A)
Table 02: Global Medical Device Labeling Market Forecast Value (US$ Mn), by Label Type 2023(E)-2033(F)
Table 03: Global Medical Device Labeling Market Historic Volume (Square Meters), by Label Type 2018(H)-2022(A)
Table 04: Global Medical Device Labeling Market Forecast Volume (Square Meters), by Label Type 2023(E)-2033(F)
Table 05: Global Medical Device Labeling Market Historic Value (US$ Mn), by Material Type 2018(H)-2022(A)
Table 06: Global Medical Device Labeling Market Forecast Value (US$ Mn), by Material Type2023(E)-2033(F)
Table 07: Global Medical Device Labeling Market Historic Volume (Square Meters), by Material Type2018(H)-2022(A)
Table 08: Global Medical Device Labeling Market Forecast Volume (Square Meters), by Material Type2023(E)-2033(F)
Table 09: Global Medical Device Labeling Market Historic Value (US$ Mn), by Application 2018(H)-2022(A)
Table 10: Global Medical Device Labeling Market Forecast Value (US$ Mn), by Application 2023(E)-2033(F)
Table 11: Global Medical Device Labeling Market Historic Volume (Square Meters), by Application 2018(H)-2022(A)
Table 12: Global Medical Device Labeling Market Forecast Volume (Square Meters), by Application 2023(E)-2033(F)
Table 13: Global Medical Device Labeling Market Historic Value (US$ Mn), By Region 2018(H)-2022(A)
Table 14: Global Medical Device Labeling Market Forecast Value (US$ Mn), By Region 2023(E)-2033(F)
Table 15: Global Medical Device Labeling Market Historic Volume (Square Meters), By Region 2018(H)-2022(A)
Table 16: Global Medical Device Labeling Market Forecast Volume (Square Meters), By Region 2023(E)-2033(F)
Table 17: North America Medical Device Labeling Market Historic Value (US$ Mn), by Label Type 2018(H)-2022(A)
Table 18: North America Medical Device Labeling Market Forecast Value (US$ Mn), by Label Type 2023(E)-2033(F)
Table 19: North America Medical Device Labeling Market Historic Volume (Square Meters), by Label Type 2018(H)-2022(A)
Table 20: North America Medical Device Labeling Market Forecast Volume (Square Meters), by Label Type 2023(E)-2033(F)
Table 21: North America Medical Device Labeling Market Historic Value (US$ Mn), by Material Type 2018(H)-2022(A)
Table 22: North America Medical Device Labeling Market Forecast Value (US$ Mn), by Material Type 2023(E)-2033(F)
Table 23: North America Medical Device Labeling Market Historic Volume (Square Meters), by Material Type 2018(H)-2022(A)
Table 24: North America Medical Device Labeling Market Forecast Volume (Square Meters), by Material Type2023(E)-2033(F)
Table 25: North America Medical Device Labeling Market Historic Value (US$ Mn), by Application2018(H)-2022(A)
Table 26: North America Medical Device Labeling Market Forecast Value (US$ Mn), by Application 2023(E)-2033(F)
Table 27: North America Medical Device Labeling Market Historic Volume (Square Meters), by Application2018(H)-2022(A)
Table 28: North America Medical Device Labeling Market Forecast Volume (Square Meters), by Application2023(E)-2033(F)
Table 29: North America Medical Device Labeling Market Historic Value (US$ Mn), By Country 2018(H)-2022(A)
Table 30: North America Medical Device Labeling Market Forecast Value (US$ Mn), By Country 2023(E)-2033(F)
Table 31: North America Medical Device Labeling Market Historic Volume (Square Meters), By Country 2018(H)-2022(A)
Table 32: North America Medical Device Labeling Market Forecast Volume (Square Meters), By Country 2023(E)-2033(F)
Table 33: Latin America Medical Device Labeling Market Historic Value (US$ Mn), by Label Type 2018(H)-2022(A)
Table 34: Latin America Medical Device Labeling Market Forecast Value (US$ Mn), by Label Type 2023(E)-2033(F)
Table 35: Latin America Medical Device Labeling Market Historic Volume (Square Meters), by Label Type 2018(H)-2022(A)
Table 36: Latin America Medical Device Labeling Market Forecast Volume (Square Meters), by Label Type 2023(E)-2033(F)
Table 37: Latin America Medical Device Labeling Market Historic Value (US$ Mn), by Material Type2018(H)-2022(A)
Table 38: Latin America Medical Device Labeling Market Forecast Value (US$ Mn), by Material Type2023(E)-2033(F)
Table 39: Latin America Medical Device Labeling Market Historic Volume (Square Meters), by Material Type2018(H)-2022(A)
Table 40: Latin America Medical Device Labeling Market Forecast Volume (Square Meters), by Material Type2023(E)-2033(F)
Table 41: Latin America Medical Device Labeling Market Historic Value (US$ Mn), by Application 2018(H)-2022(A)
Table 42: Latin America Medical Device Labeling Market Forecast Value (US$ Mn), by Application 2023(E)-2033(F)
Table 43: Latin America Medical Device Labeling Market Historic Volume (Square Meters), by Application 2018(H)-2022(A)
Table 44: Latin America Medical Device Labeling Market Forecast Volume (Square Meters), by Application 2023(E)-2033(F)
Table 45: Latin America Medical Device Labeling Market Historic Value (US$ Mn), By Country 2018(H)-2022(A)
Table 46: Latin America Medical Device Labeling Market Forecast Value (US$ Mn), By Country 2023(E)-2033(F)
Table 47: Latin America Medical Device Labeling Market Historic Volume (Square Meters), By Country 2018(H)-2022(A)
Table 48: Latin America Medical Device Labeling Market Forecast Volume (Square Meters), By Country 2023(E)-2033(F)
Table 49: Europe Medical Device Labeling Market Historic Value (US$ Mn), by Label Type 2018(H)-2022(A)
Table 50: Europe Medical Device Labeling Market Forecast Value (US$ Mn), by Label Type 2023(E)-2033(F)
Table 51: Europe Medical Device Labeling Market Historic Volume (Square Meters), by Label Type 2018(H)-2022(A)
Table 52: Europe Medical Device Labeling Market Forecast Volume (Square Meters), by Label Type 2023(E)-2033(F)
Table 53: Europe Medical Device Labeling Market Historic Value (US$ Mn), by Material Type2018(H)-2022(A)
Table 54: Europe Medical Device Labeling Market Forecast Value (US$ Mn), by Material Type2023(E)-2033(F)
Table 55: Europe Medical Device Labeling Market Historic Volume (Square Meters), by Material Type2018(H)-2022(A)
Table 56: Europe Medical Device Labeling Market Forecast Volume (Square Meters), by Material Type2023(E)-2033(F)
Table 57: Europe Medical Device Labeling Market Historic Value (US$ Mn), by Application2018(H)-2022(A)
Table 58: Europe Medical Device Labeling Market Forecast Value (US$ Mn), by Application2023(E)-2033(F)
Table 59: Europe Medical Device Labeling Market Historic Volume (Square Meters), by Application2018(H)-2022(A)
Table 60: Europe Medical Device Labeling Market Forecast Volume (Square Meters), by Application2023(E)-2033(F)
Table 61: Europe Medical Device Labeling Market Historic Value (US$ Mn), By Country 2018(H)-2022(A)
Table 62: Europe Medical Device Labeling Market Forecast Value (US$ Mn), By Country 2023(E)-2033(F)
Table 63: Europe Medical Device Labeling Market Historic Volume (Square Meters), By Country 2018(H)-2022(A)
Table 64: Europe Medical Device Labeling Market Forecast Volume (Square Meters), By Country 2023(E)-2033(F)
Table 65: Asia Pacific Medical Device Labeling Market Historic Value (US$ Mn), by Label 2018(H)-2022(A)
Table 66: Asia Pacific Medical Device Labeling Market Forecast Value (US$ Mn), by Label 2023(E)-2033(F)
Table 67: Asia Pacific Medical Device Labeling Market Historic Volume (Square Meters), by Label Type 2018(H)-2022(A)
Table 68: Asia Pacific Medical Device Labeling Market Forecast Volume (Square Meters), by Label Type 2023(E)-2033(F)
Table 69: Asia Pacific Medical Device Labeling Market Historic Value (US$ Mn), by Material Type 2018(H)-2022(A)
Table 70: Asia Pacific Medical Device Labeling Market Forecast Value (US$ Mn), by Material Type 2023(E)-2033(F)
Table 71: Asia Pacific Medical Device Labeling Market Historic Volume (Square Meters), by Material Type 2018(H)-2022(A)
Table 72: Asia Pacific Medical Device Labeling Market Forecast Volume (Square Meters), by Material Type2023(E)-2033(F)
Table 73: Asia Pacific Medical Device Labeling Market Historic Value (US$ Mn), by Application 2018(H)-2022(A)
Table 74: Asia Pacific Medical Device Labeling Market Forecast Value (US$ Mn), by Application 2023(E)-2033(F)
Table 75: Asia Pacific Medical Device Labeling Market Historic Volume (Square Meters), by Application 2018(H)-2022(A)
Table 76: Asia Pacific Medical Device Labeling Market Forecast Volume (Square Meters), by Application 2023(E)-2033(F)
Table 77: Asia Pacific Medical Device Labeling Market Historic Value (US$ Mn), By Country 2018(H)-2022(A)
Table 78: Asia Pacific Medical Device Labeling Market Forecast Value (US$ Mn), By Country 2023(E)-2033(F)
Table 79: Asia Pacific Medical Device Labeling Market Historic Volume (Square Meters), By Country 2018(H)-2022(A)
Table 80: Asia Pacific Medical Device Labeling Market Forecast Volume (Square Meters), By Country 2023(E)-2033(F)
Table 81: MEA Medical Device Labeling Market Historic Value (US$ Mn), by Label Type 2018(H)-2022(A)
Table 82: MEA Medical Device Labeling Market Forecast Value (US$ Mn), by Label Type 2023(E)-2033(F)
Table 83: MEA Medical Device Labeling Market Historic Volume (Square Meters), by Label Type 2018(H)-2022(A)
Table 84: MEA Medical Device Labeling Market Forecast Volume (Square Meters), by Label Type 2023(E)-2033(F)
Table 85: MEA Medical Device Labeling Market Historic Value (US$ Mn), by Material Type 2018(H)-2022(A)
Table 86: MEA Medical Device Labeling Market Forecast Value (US$ Mn), by Material Type2023(E)-2033(F)
Table 87: MEA Medical Device Labeling Market Historic Volume (Square Meters), by Material Type 2018(H)-2022(A)
Table 88: MEA Medical Device Labeling Market Forecast Volume (Square Meters), by Material Type 2023(E)-2033(F)
Table 89: MEA Medical Device Labeling Market Historic Value (US$ Mn), by Application 2018(H)-2022(A)
Table 90: MEA Medical Device Labeling Market Forecast Value (US$ Mn), by Application 2023(E)-2033(F)
Table 91: MEA Medical Device Labeling Market Historic Volume (Square Meters), by Application 2018(H)-2022(A)
Table 92: MEA Medical Device Labeling Market Forecast Volume (Square Meters), by Application 2023(E)-2033(F)
Table 93: MEA Medical Device Labeling Market Historic Value (US$ Mn), By Country 2018(H)-2022(A)
Table 94: MEA Medical Device Labeling Market Forecast Value (US$ Mn), By Country 2023(E)-2033(F)
Table 95: MEA Medical Device Labeling Market Historic Volume (Square Meters), By Country 2018(H)-2022(A)
Table 96: MEA Medical Device Labeling Market Forecast Volume (Square Meters), By Country 2023(E)-2033(F)
List of Figures
Figure 01: Global Medical Device Labeling Market Share Analysis by Label Type, 2023E & 2033F
Figure 02: Global Medical Device Labeling Market Attractiveness Analysis by Label Type, 2023E-2033F
Figure 03: Global Medical Device Labeling Market Y-o-Y Analysis by Label Type, 2018H-2033F
Figure 04: Global Medical Device Labeling Market Share Analysis by Material Type, 2023E & 2033F
Figure 05: Global Medical Device Labeling Market Attractiveness Analysis by Material Type, 2023E-2033F
Figure 06: Global Medical Device Labeling Market Y-o-Y Analysis by Material Type, 2018H-2033F
Figure 07: Global Medical Device Labeling Market Share Analysis by Application, 2023E & 2033F
Figure 08: Global Medical Device Labeling Market Attractiveness Analysis by Application, 2023E-2033F
Figure 09: Global Medical Device Labeling Market Y-o-Y Analysis by Application, 2018H-2033F
Figure 10: Global Medical Device Labeling Market Share Analysis by Region, 2023E & 2033F
Figure 11: Global Medical Device Labeling Market Attractiveness Analysis by Region, 2023E-2033F
Figure 12: Global Medical Device Labeling Market Y-o-Y Analysis by Region, 2018H-2033F
Figure 13: North America Medical Device Labeling Market Value Share Analysis by Label Type 2023(E)
Figure 14: North America Medical Device Labeling Market Value Share Analysis by Material Type 2023(E)
Figure 15: North America Medical Device Labeling Market Attractiveness Analysis by Application, 2023E-2033F
Figure 16: North America Medical Device Labeling Market Value Share Analysis by Country 2023(E)
Figure 17: Latin America Medical Device Labeling Market Value Share Analysis by Label Type 2023(E)
Figure 18: Latin America Medical Device Labeling Market Value Share Analysis by Material Type 2023(E)
Figure 19: Latin America Medical Device Labeling Market Attractiveness Analysis by Application, 2023E-2033F
Figure 20: Latin America Medical Device Labeling Market Value Share Analysis by Country 2023(E)
Figure 21: Europe Medical Device Labeling Market Value Share Analysis by Label Type 2023(E)
Figure 22: Europe Medical Device Labeling Market Value Share Analysis by Material Type 2023(E)
Figure 23: Europe Medical Device Labeling Market Attractiveness Analysis by Application, 2023E-2033F
Figure 24: Europe Medical Device Labeling Market Value Share Analysis by Country 2023(E)
Figure 25: Asia Pacific Medical Device Labeling Market Value Share Analysis by Label Type 2023(E)
Figure 26: Asia Pacific Medical Device Labeling Market Value Share Analysis by Material Type 2023(E)
Figure 27: Asia Pacific Medical Device Labeling Market Attractiveness Analysis by Application, 2023E-2033F
Figure 28: Asia Pacific Medical Device Labeling Market Value Share Analysis by Country 2023(E)
Figure 29: MEA Medical Device Labeling Market Value Share Analysis by Label Type 2023(E)
Figure 30: MEA Medical Device Labeling Market Value Share Analysis by Material Type 2023(E)
Figure 31: MEA Medical Device Labeling Market Attractiveness Analysis by Application, 2023E-2033F
Figure 32: MEA Medical Device Labeling Market Value Share Analysis by Country 2023(E)
Figure 33: U.S. Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 34: U.S. Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 35: U.S. Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 36: Canada Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 37: Canada Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 38: Canada Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 39: Brazil Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 40: Brazil Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 41: Brazil Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 42: Mexico Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 43: Mexico Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 44: Mexico Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 45: Germany Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 46: Germany Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 47: Germany Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 48: Spain Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 49: Spain Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 50: Spain Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 51: France Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 52: France Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 53: France Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 54: U.K. Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 55: U.K. Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 56: U.K. Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 57: Italy Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 58: Italy Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 59: Italy Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 60: Russia Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 61: Russia Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 62: Russia Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 63: China Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 64: China Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 65: China Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 66: India Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 67: India Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 68: India Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 69: Japan Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 70: Japan Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 71: Japan Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 72: GCC Countries Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 73: GCC Countries Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 74: GCC Countries Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F
Figure 75: South Africa Medical Device Labeling Market Value Share Analysis, by Label Type, 2023E
Figure 76: South Africa Medical Device Labeling Market Value Share Analysis, by Material Type, 2023E
Figure 77: South Africa Medical Device Labeling Market Value Share Analysis, by Application, 2023E & 2033F